(UroToday.com) The 2023 SUO annual meeting included a session on prostate cancer, featuring a presentation by Dr. Nimrod Barashi discussing the association of the Decipher® genomic classifier on initial prostate biopsy and progression from active surveillance to treatment. Active surveillance is currently the recommended treatment for very low- and low-risk prostate cancer and is also being more frequently used for favorable intermediate risk prostate cancer in select patients.
Clinical tools such as prostate multiparametric MRI and genomic classifiers such as the Decipher® genomic classifier are recommended to risk stratify newly diagnosed prostate cancer patients in the pre-treatment setting. The aim of this study presented at SUO 2023 was to examine the relationship between pre-treatment parameters such as PSA, biopsy Gleason Grade Group, MRI Prostate Imaging-Reporting and Data System (PI-RADS) score, and Decipher® genomic classifier on progression to treatment in patients currently on active surveillance for prostate cancer.
This study retrospectively examined patients at a single institution who underwent Decipher® genomic classifier testing on prostate biopsy tissue (n = 888) between December 2016 and March 2023. Data regarding PSA, PI-RADS lesion on MRI, biopsy Gleason Grade Group, Decipher® genomic classifier, and treatment modality were collected. Among these patients, 235 were placed on active surveillance initially, and ultimately 88 patients progressed to treatment. Dr. Barashi and colleagues performed a multivariable Cox proportional hazards model with time to treatment as the outcome and PSA, PI-RADS score, and Decipher® genomic classifier as potential predictors.
This cohort had a mean PSA of 7.4 ng/mL and a mean Decipher® genomic classifier score of 0.38, with 25.5% of these patients (n = 60) having PIRADS 1-2 lesions on MRI, 14% (n = 33) with PIRADS 3 lesions, and 60.4% (n = 142) with PIRADS 4-5 lesions. Overall, 40% of these patients (n = 94) had Gleason Grade Group 1, 48.1% (n = 113) had Gleason Grade Group 2, and 11.9% (n = 28) had Gleason Grade Group 3 or higher on biopsy. A Decipher® genomic classifier above 0.4 – 0.6 (vs < 0.4: HR 2.61, 95% CI 1.56 – 4.38; p < 0.001) and >0.6 (vs <0.4: HR 2.58, 95% CI 1.40 – 4.75; p = 0.002) was associated with progression to treatment while on active surveillance, whereas having a PI-RADS 3 lesion (p = 0.721) and having a PI-RADS 4-5 lesion (p = 0.057) were not associated with progression to treatment: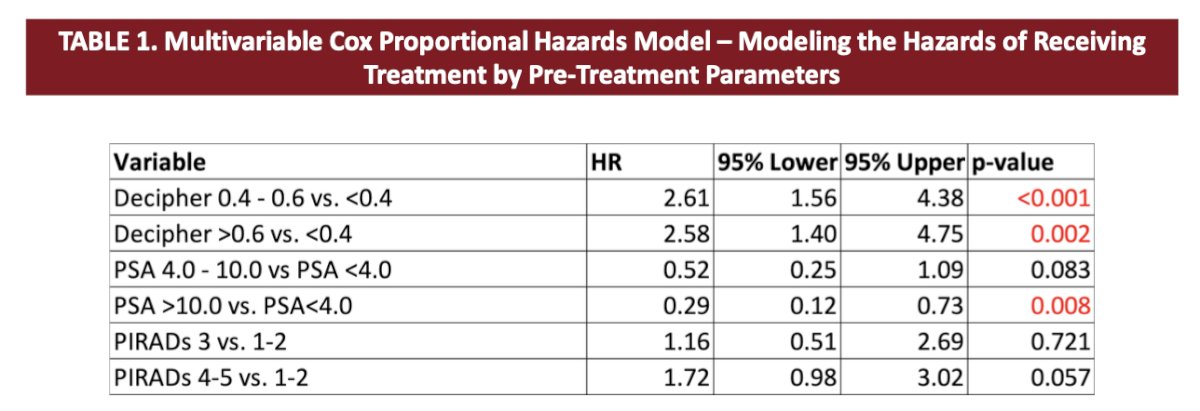
Survival curves showed that increased Decipher® genomic classifier was associated with decreased freedom from treatment (p = 0.0026), whereas there was no association with PI-RADS score (p = 0.088):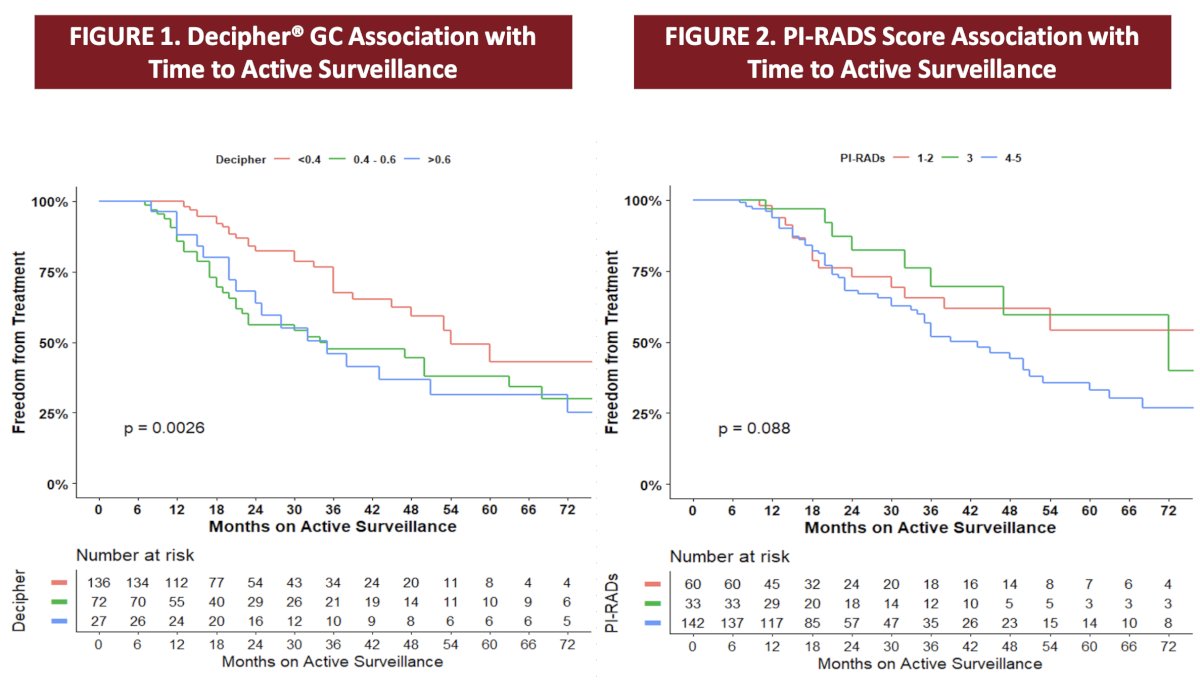
Dr. Barashi concluded his presentation discussing the association of the Decipher® genomic classifier on initial prostate biopsy and progression from active surveillance to treatment with the following take-home points:
- Tumor-based molecular assays such as Decipher® genomic classifier are included in the NCCN guidelines for localized prostate cancer management
- Decipher® genomic classifier has been shown to independently predict adverse pathology with very low-, low- and intermediate-risk prostate cancer
- Previous studies have shown the association of Decipher® genomic classifier score with progression to treatment on active surveillance without incorporating multiparametric MRI data
- This study found that an increased Decipher® genomic classifier is an independent predictor of progression to treatment in a cohort of patients on active surveillance, while multiparametric MRI (PI-RADS) is not associated with progression
- Future directions include investigating the GRID database to see if there are certain gene expressions or signatures that are associated with progression to treatment while on active surveillance
Presented by: Nimrod S. Barashi, MD, Washington University School of Medicine, St. Louis, MO
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 Society of Urologic Oncology (SUO) Annual Meeting, Washington, D.C., Tues, Nov 28 – Fri, Dec 1, 2023.


