(UroToday.com) The 2023 SUO annual meeting included a session on urothelial cancer, featuring a presentation by Dr. Katie Murray discussing chemoablation and renal preservation as the future for management of upper tract urothelial carcinoma (UTUC). Dr. Murray notes that in 2023 UTUC is still a rare malignancy encompassing 6,000 to 7,000 cases per year in the United States (and by comparison, testicular cancer is 8,000 to 10,000 cases per year). Smoking is the most common risk factor, increasing the risk of UTUC 2-4x that above non-smokers. Additional risk factors include a history of bladder cancer, radiation exposure, and chemical exposure (aromatic hydrocarbons-petroleum/paint/textile, cyclophosphamide, ifosfamide, arsenic). Finally, there is a hereditary component to UTUC, specifically Lynch syndrome, which is the 3rd most common cancer in this syndrome.
Importantly, the AUA/SUO released the 2023 guidelines for the diagnosis and management of non-metastatic upper tract urothelial carcinoma in order to help guide management.1 Secondly, Dr. Murray emphasized that the goal of this specific talk is to highlight kidney sparing. The AUA/SUO guideline statement 9 states “At time of identified UTUC, clinicians should perform a standardized assessment documenting clinically meaningful endoscopic and radiographic features to facilitate clinical staging and risk assessment (strong recommendation; evidence level grade B).” Specifically, for ureteroscopy, a standardized report should include:
- Sites of involvement
- Number of tumors
- Tumor appearance (descriptive)
- Size of largest tumor (compared to the scope, basket, biopsy forceps, etc)
- Retrograde pyelogram with description
- Volume measurement of the upper tract
Dr. Murray emphasized that the reason to document and risk assess these patients is to assess each patient for renal preservation, which is a risk based management decision.
AUA/SUO guideline statement 10 states “Following standardized assessment, clinicians should risk-stratify patients as “low” or “high” risk for invasive disease (pT2 or greater) based on obtained endoscopic, cytologic, pathologic, and radiographic findings. Further stratification into favorable and unfavorable risk groups should then be based on standard identified features (strong recommendation; evidence level Grade B).” As such, biopsy alone is not enough for these patients. The following table offers a risk stratification and management algorithm:

Ablative techniques for renal preservation include endoscopic ablations, percutaneous resections, chemoablation, and new technologies for tumor ablation (mechanical and chemical tumor destruction). For endoscopic ablations, this can be technically challenging, there may be residual tumor, intravesical recurrences occur, primary recurrences are common, and disease progression can occur. At the same time as cancer control, there are other goals in organ sparing options for UTUC, which include: increased tolerability, increased efficacy, increased durability, and decreased morbidity.
In a recent meta-analysis assessing chemoablation for urothelial carcinoma, including 23 studies comprising 1,199 patients.2 Among patients treated with weekly administration of any agent, the pooled CR rates at initial assessment were 50.9% (95% CI 45.9-55.9) for the marker lesion and 47.5% (95% CI 36.5-58.7) for well-selected NMIBC (ie. small tumors and/or a small number of tumors). Novel regimens for chemoablation such as mitomycin C-gel (70.6%, 95% CI 60.1-79.3) and an intensive mitomycin C regimen (64.7%, 95% CI: 56.2-72.3) provided better complete response rates in well-selected NMIBC patients:
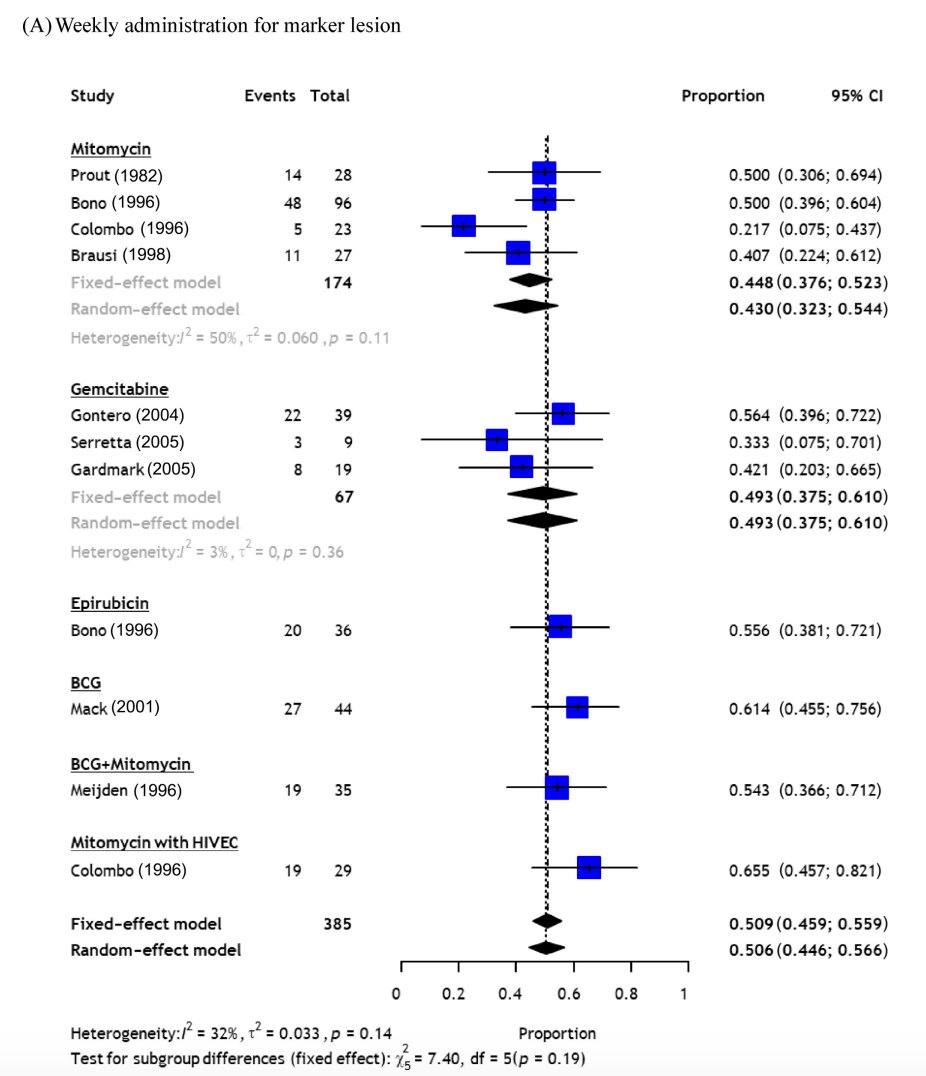
With regards to endoluminal treatments, McElree et al.3 assessed 41 renal units that were treated with sequential infusions of 1 g gemcitabine in a 50 ml bag of normal saline, followed by 37.5 mg docetaxel in a 50 ml saline bag. Treatment drug was hung 30 to 35 cm above the flank level, connected to the instillation tube via standard intravenous tubing, and 5 cc was rapidly run in to fill the renal pelvis followed by a constant gravity drip at a rate of 1 drip every 3 to 4 seconds for a total of 90 minutes. Induction treatments took place once weekly for 6 weeks. Recurrence-free survival was 76%, 54%, and 40% at 1, 2, and 3 years, respectively. The 3-year progression-free and overall survival were 75% and 75%, respectively, with five patients dying from urothelial carcinoma:

There are several potential questions/issues for dwell time for chemoablation in the upper urinary tract:
- How do we instill? What is the route of administration to allow for intensive regimens
- Can you make the upper urinary tract a temporary storage organ like the bladder or overcome the fact that it is a transport organ? Perhaps this is an opportunity for drug eluting implants and/or reverse thermal hydrogel technology
Dr. Murray then discussed the OLYMPUS trial, first published in 2020.4 This trial was an open-label, single-arm, phase 3 trial of primary chemoablation of low-grade UTUC using UGN-101, a mitomycin-containing reverse thermal gel. Enrolled patients received six once-weekly instillations of UGN-101 as an induction course, which was administered via retrograde instillation with ureteral catheterization. Of the 71 patients who received at least one dose of treatment, 42 patients (59%, 95% CI 47-71%) had a complete response at the time of primary disease evaluation. Of the remainder, 8 (11%) had a partial response, 12 (17%) had no response, 6 (8%) had newly diagnosed high-grade disease, and 3 (4%) had an indeterminate response. Despite these promising results, toxicity was not insignificant: 67 patients (94%) experienced adverse events and 26 (37%) patients experienced severe adverse events. 60 patients (85%) had adverse events that were deemed treatment-related and 19 (27%) had severe treatment related events. 19 patients (27%) discontinued treatment due to adverse events both in the initial 6-week treatment period (9 patients, 13%) and during maintenance (10 patients, 14%). Among adverse events of particular interest, renal functional impairment was noted in 14 patients (20%). There was also a significant burden of urinary tract morbidity: among 71 patients who received at least one dose of study medication, 48 patients (68%) had an adverse event related to the urinary system including 11 (23%) who did not require surgical intervention, 24 (50%) who required transient stent placement, 11 (23%) who required long-term stent placement (still in place at the time of data cut-off), and 2 (4%) who required nephroureterectomy due the need for permanent drainage as a result of ureteral stenosis.
In the final report of OLYMPUS published in 2023,5 41 (58%) had complete response to induction therapy and consented to long-term follow-up. Among these patients, 56% remained in complete response after 12 months (95% CI 40, 72), comprising 6/12 (50%) who did not receive any maintenance instillations and 17/29 (59%) who received ≥1 maintenance instillation. Kaplan-Meier analysis of durability was estimated as 82% (95% CI 66, 91) at 12 months:

Ureteric stenosis was the most frequently reported treatment emergent adverse events (31/71, 44%), with an increasing number of instillations appearing to be associated with increased incidence of urinary treatment emergent adverse events.
Dr. Murray then highlighted AUA/SUO statements 12-17:
- Statement 12: “Clinicians should provide patients with a description of the short- and long-term risk associated with recommended diagnostic and therapeutic options. This includes the need for endoscopic follow-up, clinically significant strictures, toxicities associated with surgical treatment and side effects from neoadjuvant and adjuvant therapies (Clinical Principle).”
- Statement 13: “Tumor ablation should be the initial management options for patients with low risk favorable UTUC (Strong Recommendation; Evidence Level: Grade B).”
- Statement 14 “Tumor ablation may be the initial management offered to patients with low risk unfavorable UTUC and select high risk favorable disease who have low-volume tumors or cannot undergo radical nephroureterectomy. (Conditional Recommendation; Evidence Level: Grade C).”
- Statement 15 “Tumor ablation may be accomplished via a retrograde or antegrade percutaneous approach and repeat endoscopic ablation should be performed within 3 months. (Expert Opinion).”
- Statement 16 “Following ablation of UTUC tumors and after confirming no perforation of the bladder or upper tract, clinicians may instill adjuvant pelvicalyceal chemotherapy (Conditional Recommendation; Evidence Level: Grade C) or intravesical chemotherapy (Expert Opinion) to decrease the risk of urothelial cancer recurrence”
- Statement 17 “Pelvicalyceal therapy with BCG may be offered to patients with High Risk Favorable UTUC after complete tumor ablation or patients with upper tract CIS. (Expert Opinion).”
Finally, Dr. Murray highlighted statement 32 of the guidelines, which is specific to follow-up in patients with renal preservation “Low-risk patients managed with kidney sparing treatment should undergo a follow-up cystoscopy and upper tract endoscopy within one to three months to confirm successful treatment. Once confirmed, these patients should undergo continued cystoscopic surveillance of the bladder at least every six to nine months for the first two years and then at least annually thereafter. Endoscopy should be repeated at six months and one year. Upper tract imaging should be performed at least every six to nine months for two years, then annually up to five years. surveillance after five years in the absence of recurrence should be based on shared decision- making between the patient and clinician. (Expert Opinion)”

Dr. Murray concluded her presentation discussing chemoablation and renal preservation as the future for management of UTUC by once again emphasizing that for UTUC, organ sparing is kidney sparing.
Presented by: Katie S. Murray, DO, New York University, New York, NY
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 Society of Urologic Oncology (SUO) Annual Meeting, Washington, D.C., Tues, Nov 28 – Fri, Dec 1, 2023.
References:
- Coleman JA, Clark PE, Bixler BR, et al. Diagnosis and Management of Non-Metastatic Upper Tract Urothelial Carcinoma: AUA/SUO Guideline. J Urol. 2023 Jun;209(6):1071-1081.
- Yanagisawa T, Quhal F, Kawada T, et al. A systematic review and meta-analysis of chemoablation for non-muscle-invasive bladder cancer. Eur Urol Focus. 2023 May;9(3):463-479.
- McElree IM, Belzer A, Mott SL, et al. Sequential endoluminal gemcitabine and docetaxel for the treatment of clinically non-invasive high-grade upper tract urothelial carcinoma. Urol Oncol. 2023 Oct 5;S1078-1439(23)00289-2.
- Kleinmann N, Matin SF, Pierorazio PM, et al. Primary chemoablation of low-grade upper tract urothelial carcinoma using UGN-101, a mitomycin-containing reverse thermal gel (OLYMPUS): An open-label, single-arm, phase 3 trial. Lancet Oncol 2020 Jun;21(6):776-785.
- Matin SF, Pierorazio PM, Kleinmann N, et al. Durability of response to primary chemoablation of low-grade upper tract urothelial carcinoma using UGN-101, a mitomycin-containing reverse thermal gel: OLYMPUS Trial Final Report. J Urol. 2022 Apr;207(4):779-788.


