(UroToday.com) The 2024 SUO annual meeting included a session on non muscle invasive bladder cancer, featuring a presentation by Dr. Ashish Kamat discussing BCG unresponsive cancer in 2024.
Dr. Kamat started by highlighting the changes made in the non muscle invasive bladder cancer (NMIBC) disease space from 1970 – 2018, specifically BCG’s approval in 1990 for treatment of CIS and to prevent relapse of Ta and T1 disease, the FDA’s public workshop in 2013 for clinical trial design issues for developing new therapies in NMIBC, clarification of bladder cancer disease states following treatment of patients with intravesical BCG in 2015,1 the International Bladder Cancer Group’s definitions, endpoints, and clinical trial designs for NMIBC in 2016,2 and the FDA’s guidance for industry of BCG-unresponsive NMIBC development of drugs and biologics for treatment: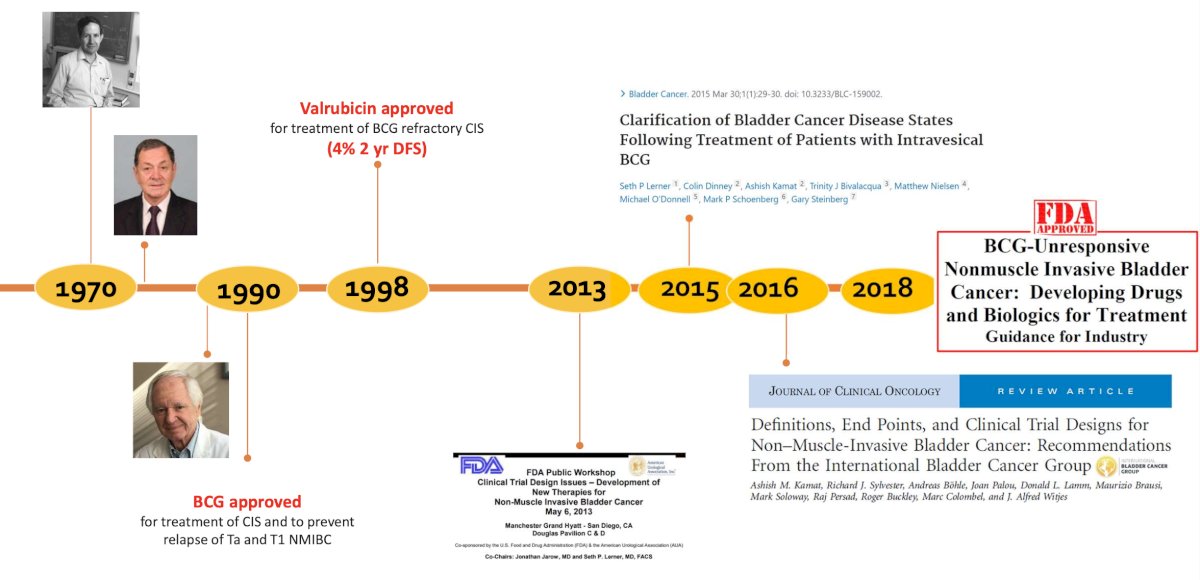
Importantly, adequate BCG therapy is defined as at least 5 of 6 doses of induction BCG and at least 2 additional doses of maintenance BCG. However, this is a definition for clinical trials and not for treatment recommendations.
Dr. Kamat then discussed several treatment regimens that are approved for BCG-unresponsive CIS. Pembrolizumab was approved based on the KEYNOTE-057 trial.3 Patients in this trial received intravenous pembrolizumab at 200 mg every 3 weeks for up to 24 months or until patients had evidence of centrally confirmed disease persistence, recurrence, or progression, or unacceptable drug-related toxicity. Patients underwent a cystoscopy and had a urine cytology performed every three months for the first two years and every six months thereafter for up to five years. There were no protocol-mandated biopsies, which were only performed in case of an abnormal cytology or cystoscopic findings. Overall, 64% of patients had CIS-only disease, and the 3-month complete response rate, defined as absence of high-risk NMIBC or progressive disease, was 40.6%. The median duration of complete response was 16.2 months: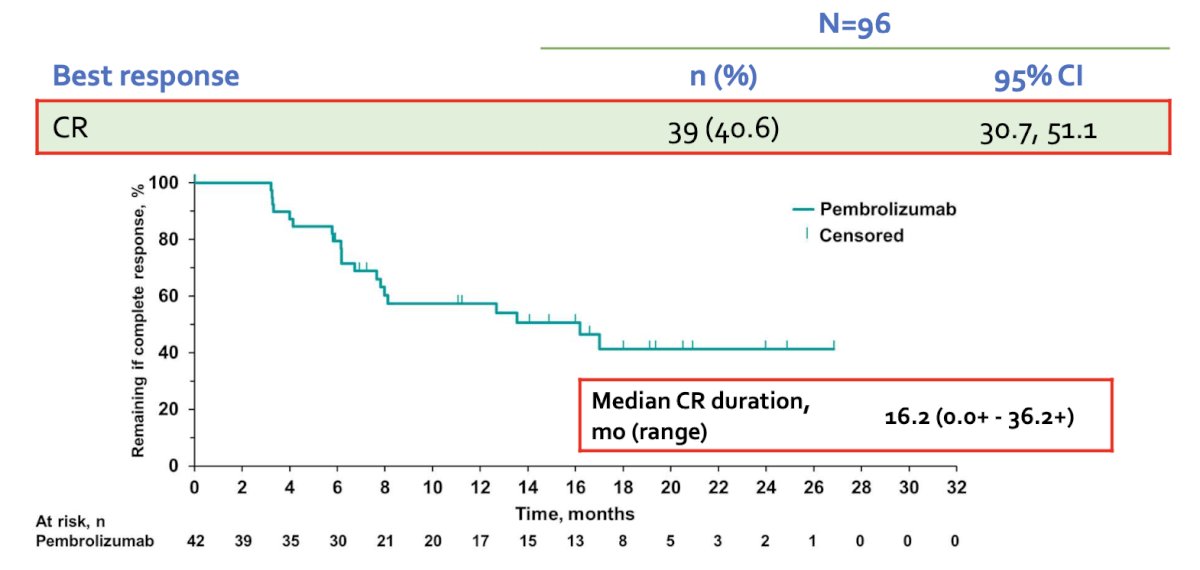
Results of Cohort B (papillary-only disease) were published in Lancet Oncology earlier in 2024.4 This cohort included 132 patients, with a median follow-up of 45.4 months, with 43% of patients having T1 disease. The 12-month disease-free survival rate (primary outcome) was 43.5% (95% CI: 35 to 52%), and the corresponding 24- and 36-month rates were 35% for both: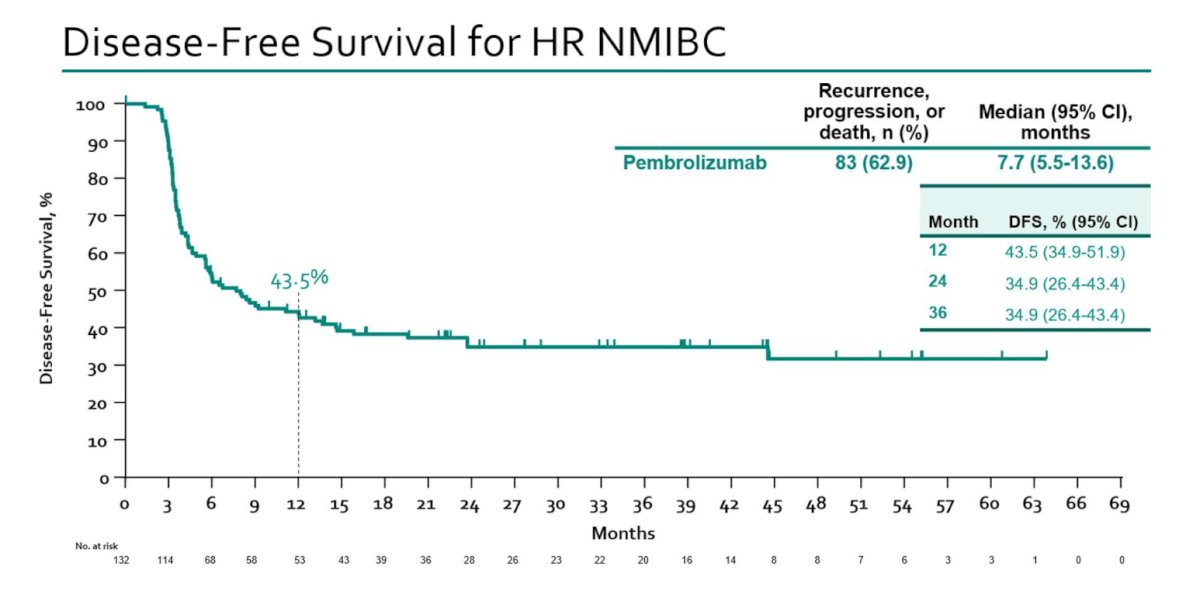
Freedom from disease progression, defined as grade or stage worsening, development of invasive or metastatic disease or death, was observed in 88.2% of patients. The one-year overall survival rate was 96.2%.
In 2021, the results of the phase III trial that led to the subsequent FDA approval of nadofaragene firadenovec were published in Lancet Oncology.5 This was a phase 3, multicenter, open-label, repeat-dose study across 33 centers in the United States of 151 patients with BCG-unresponsive NMIBC. Eligible patients received a single intravesical 75 mL dose of nadofaragene firadenovec. The protocol mandated a 5-site biopsy (dome, trigone, right and left lateral walls, posterior wall) at 12 months, and patients who were free of high-grade recurrence were offered continued treatment once every three months at the investigator's discretion. This study met its primary endpoint with 53% of patients with CIS +/- papillary disease achieving a complete response at three months, with 24% maintaining this response by 12 months. Patients with high-grade Ta/T1 tumors achieved 73% and 44% high-grade recurrence-free survivals at three and 12 months, respectively: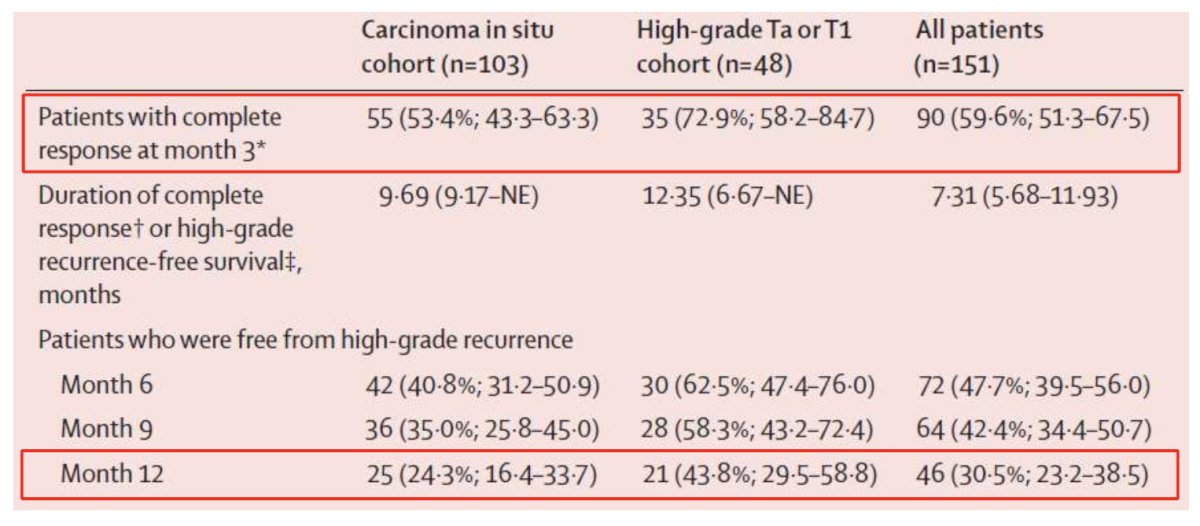
The median duration of high-grade recurrence-free survival was 12.35 months (95% CI 6.67-NE) in patients with papillary disease, and progression to muscle-invasive disease occurred in 8 patients (5.3%). With additional long-term follow-up among patients with CIS +/- Ta/T1 disease, 5.8% of participants were free of high-grade disease at 57 months, and 10.9% who had a complete response at 3 months remains free of high-grade disease at 57 months: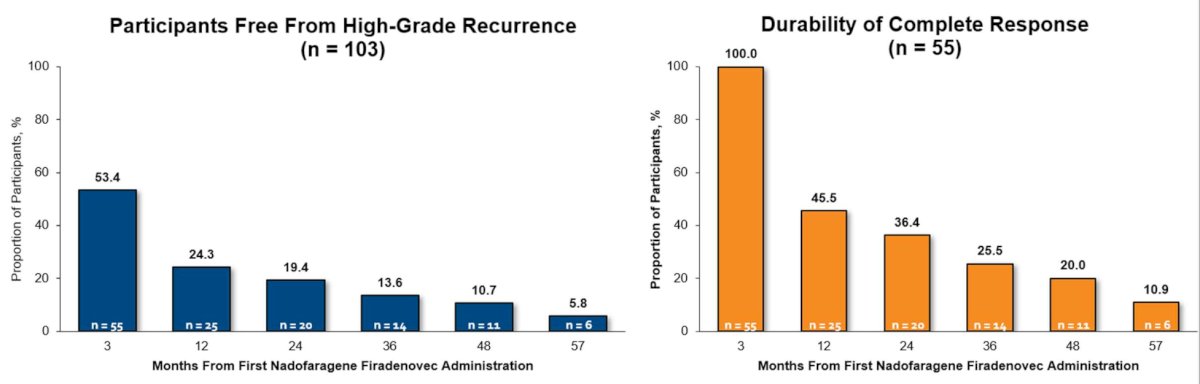
The Quilt 3032 trial6 led to the approval of N-803 (nogapendekin alfa inbakicept: ANKTIVA®), which is an interleukin-15 superagonist (IL-15), that promotes activation and proliferation of natural killer cells, CD8+ T cells and memory T cells without expanding immunosuppressive T-reg cells. N-803 synergizes with BCG to elicit durable complete responses and has recently been FDA-approved for BCG-unresponsive NMIBC CIS, with or without papillary tumors. In this trial, over a median follow-up of 23.9 months, the complete response rate was 71% and the median duration of response was 26.6 months. Based on these results, the FDA granted breakthrough approval of N-803 in April 2024: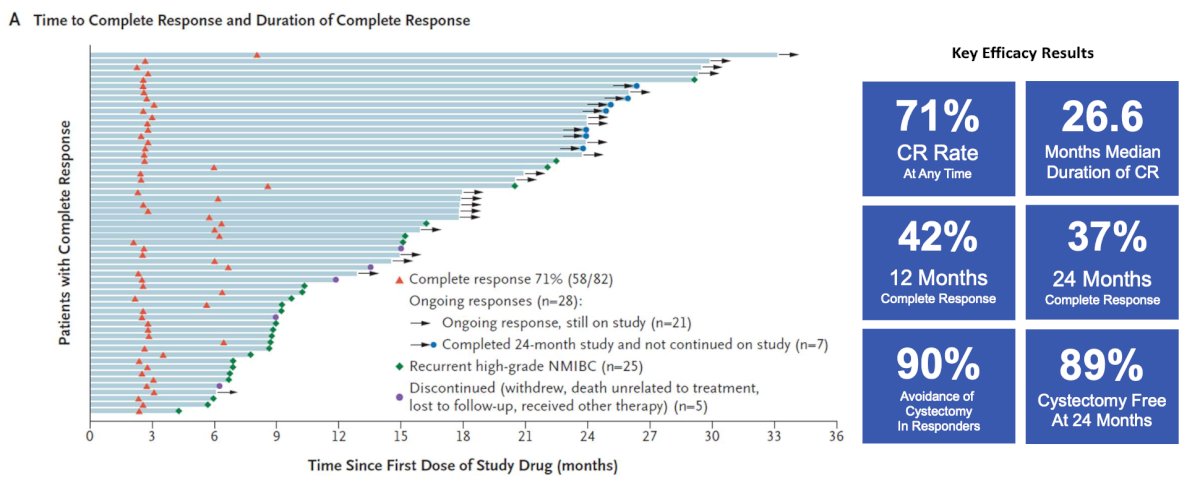
Dr. Kamat then updated the timeline from 2018 through 2025 and beyond, where we have additional new potential therapeutics on the market including TAR-200, cretostimogene +/- pembrolizumab, and detalimogene voraplasmid: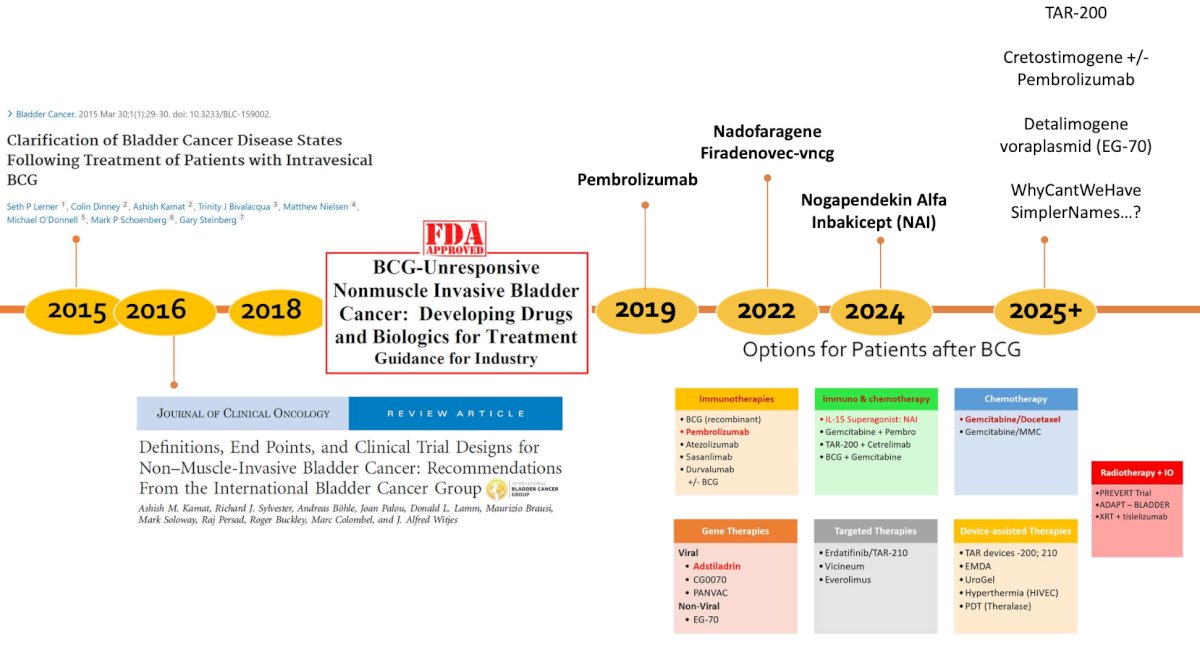
At ESMO 2024, Dr. Michiel Van der Heijden presented results of TAR-200 +/- cetrelimab and cetrelimab alone in patients with BCG-unresponsive high risk NMIBC. TAR-200 is an intravesical targeted releasing system designed to provide sustained delivery of gemcitabine in the bladder over 3 weeks. SunRISe-1 is an ongoing randomized, phase 2b study evaluating efficacy and safety of TAR-200 + cetrelimab (Cohort 1), TAR-200 alone (Cohort 2), or cetrelimab alone (Cohort 3) in patients with BCG unresponsive high-risk NMIBC ineligible for/refusing radical cystectomy. Eligible patients (≥18 years) had histologically confirmed CIS ± papillary disease (high-grade Ta, any T1) with last dose of adequate BCG ≤12 months of CIS diagnosis, and ECOG performance status 0-2. TAR-200 was dosed Q3W to week 24 then Q12W to week 96, while cetrelimab was dosed Q3W to week 78. Centrally confirmed complete response rates in Cohort 1, Cohort 2, and Cohort 3 were 68%, 84%, and 46%, respectively: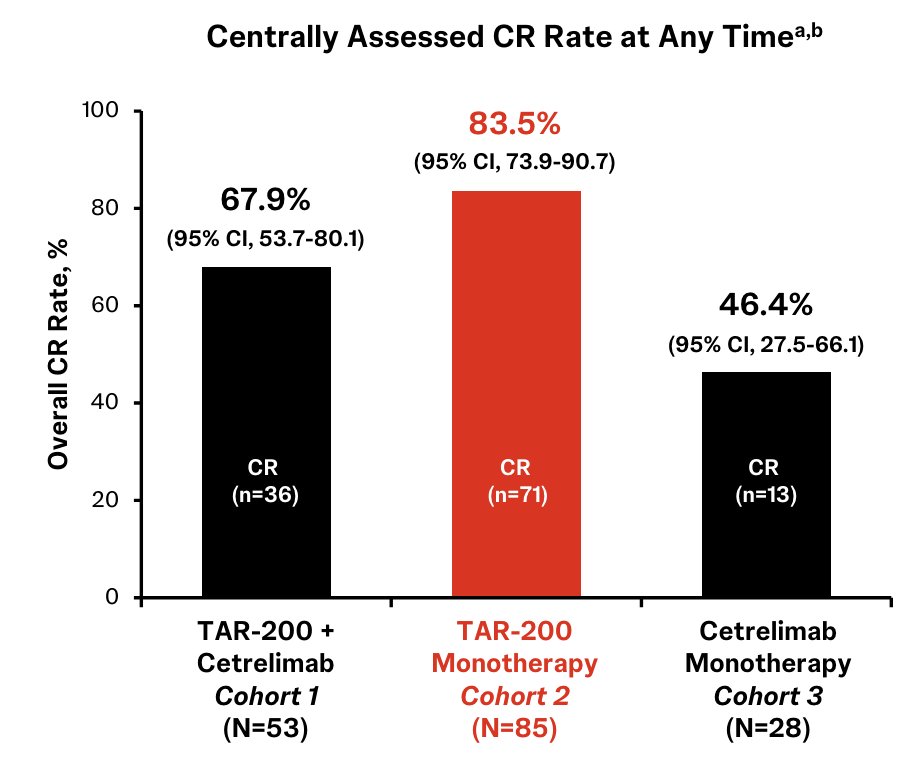
At AUA 2024, Dr. Mark Tyson presented updated results of the BOND-003 study assessing cretostimogene as monotherapy for BCG-unresponsive NMIBC. This included a 75.2% (95% CI 65% - 83%) complete response rate at any time based on central review, comparable to initial results presented at SUO 2023 (75.7%):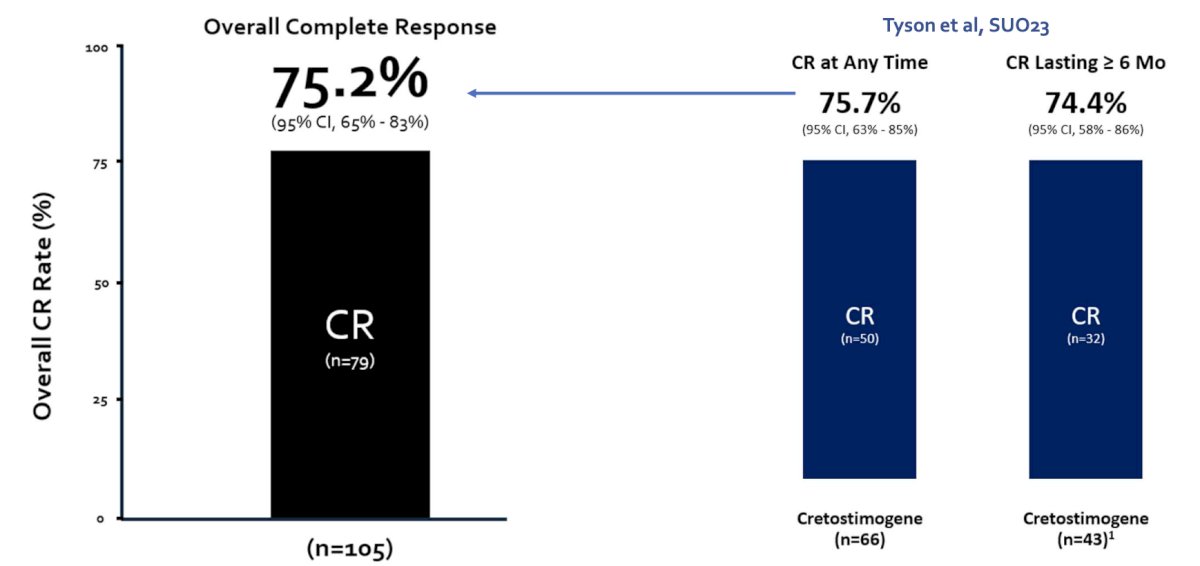
Also at AUA 2024, initial results of a phase 1/2 trial assessing EG-70 (detalimogene voraplasmid) were presented by Dr. Gordon Brown. In the Phase 1 part of the study, three escalating doses of EG-70 were assessed, administered on two schedules at study weeks 1 and 2 to BCG-unresponsive patients with CIS. Patients received either 2 or 4 doses over a 12-week period, following a 3+3 dose escalation design, with dose levels of 0.25 mg/mL, 0.8 mg/mL, and 2.5 mg/mL. After completing the 12 week study, patients showing either a complete response or stable disease were allowed to continue EG-70 for up to 3 additional 12 week cycles. In the 12 week Phase 1 trial segment, 24 patients were enrolled and received at least one dose of EG-70, totaling 175 doses administered. There were no safety concerns noted, and no dose-limiting toxicities were observed that necessitated dose de-escalation. In terms of efficacy, 22 patients were evaluable for this outcome. A total of 15/22 (68%) experienced a complete response at 3 months, with an additional 1/22 (5%) achieving complete response at some point in treatment: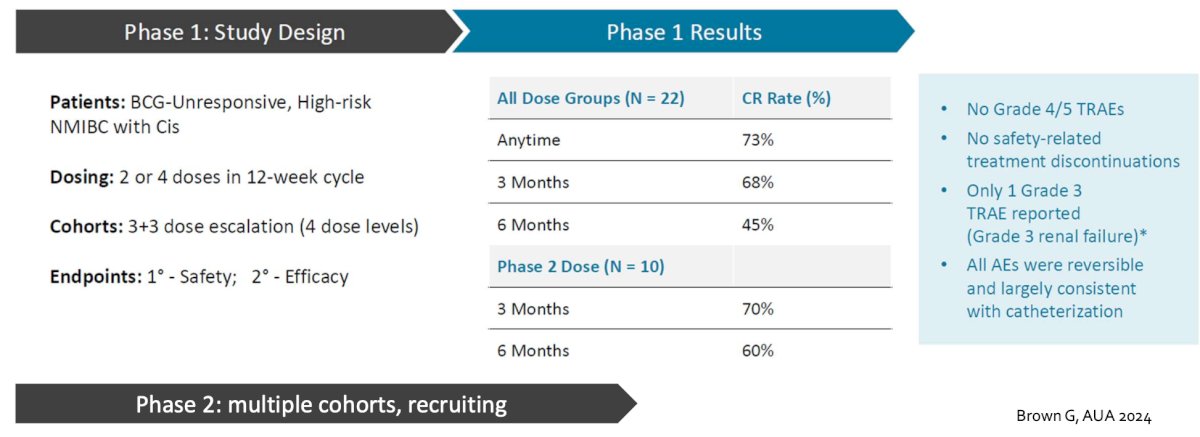
At ASCO 2023, Dr. Kamat presented initial results of the phase 1 EV-104 study assessing intravesical enfortumab vedotin. Among 6 patients across doses, there were no Grade ≥3 treatment related adverse events, treatment-related symptomatic adverse events, treatment related adverse events leading to dose reduction, or discontinuation. Additionally, 4 of 6 patients (3 at 125mg and 1 at 250mg) experienced ≥1 treatment-related adverse event of Grade 1 or 2. Of 4 patients receiving 125mg of intravesical enfortumab vedotin, 3 achieved complete response and continue in response. The fourth patient discontinued treatment due to persistent disease but remains on study: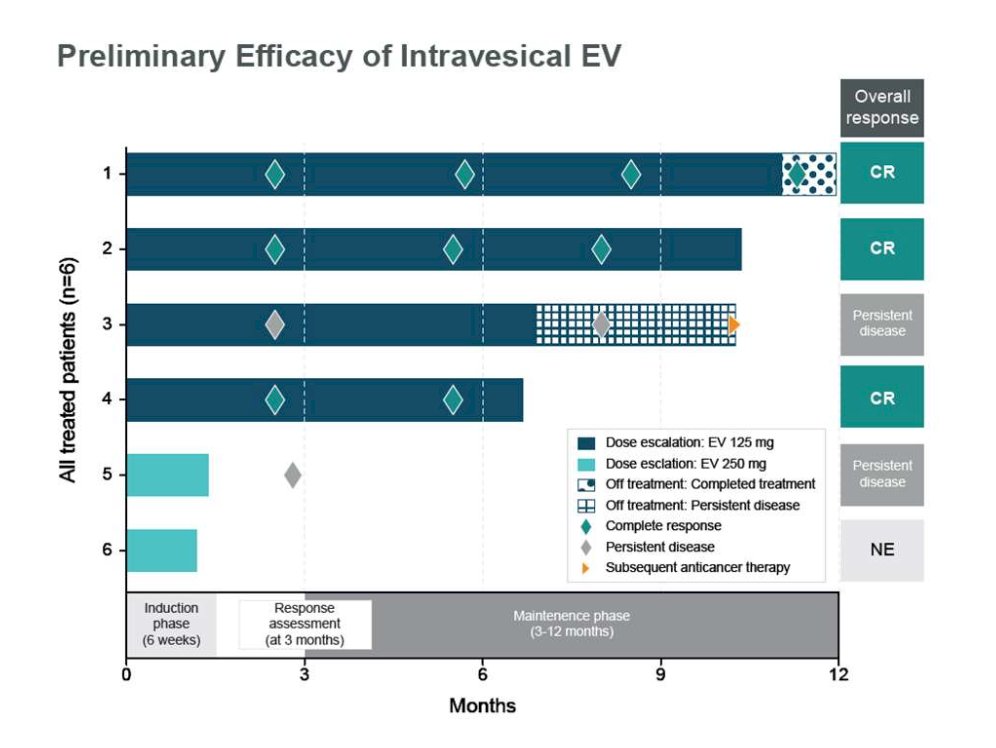
Photodynamic laser therapy (Theralase) has also recently garnered interest in this disease space. From an October 2024 press release, among 75 patients enrolled across 10 study sites (5 in Canada and 5 in the US), the complete response rate was 60.3%, with 35.6% of patients having a complete response >= 3 years: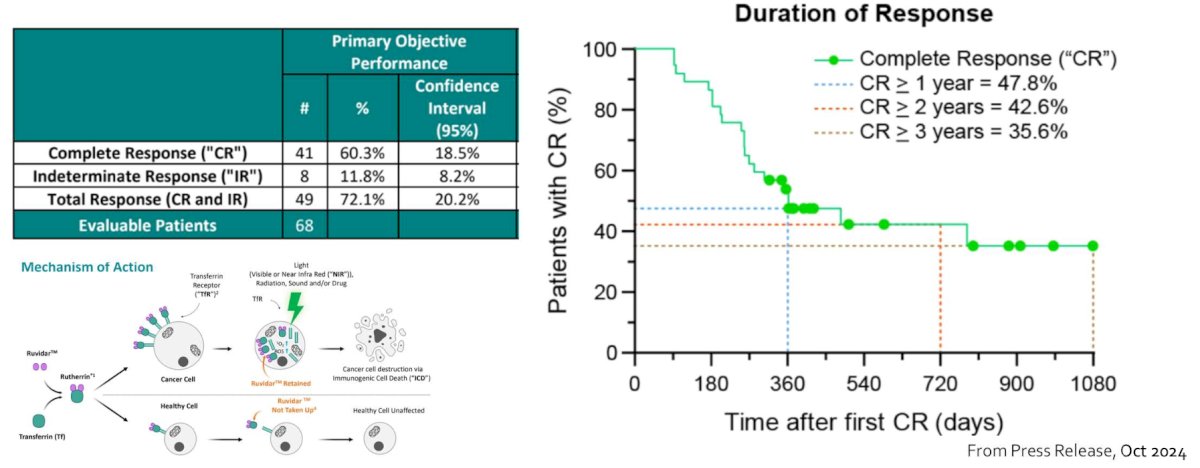
Dr. Kamat then discussed TARA-002, a lyophilized biological preparation for instillation containing cells of Streptococcus pyogenes (Group A, type 3) Su strain treated with benzylpenicillin. The chemical characteristics of TARA-002 are intended to be comparable to OK-432, which is approved in Japan and Taiwan for the treatment of several different cancers (head and neck, gastric, and lung cancer). The antitumor activity of TARA-002 and OK-432 is thought to occur by direct cytotoxicity and by stimulation of immunocompetent cells through the induction of helper T-cell type-1 cytokines, which then recruit cytotoxic T lymphocytes to tumor cells. As such, nonclinical and clinical evidence with OK-432 and TARA-002 support the ADVANCED-1 study (dose escalation Phase 1a [NCT05085977]. ADVANCED-2 is a Phase 1b/2, dose-expansion, open-label study of intravesical instillation of TARA-002 in adults with high-grade CIS NMIBC. The purpose of this study is to evaluate the safety and anti-tumor activity of TARA-002 administered intravesically for the treatment of subjects with CIS NMIBC (± Ta/T1) with active disease. The study includes 102 eligible male and female subjects ≥ 18 years of age enrolled in two cohorts based on their prior BCG experience.
In the wake of the BCG shortage over the last several years, intravesical gemcitabine + docetaxel has emerged as a combination therapy for patients with NMIBC. In 2020, Steinberg et al. [7] investigated intravesical gemcitabine/docetaxel as rescue therapy for NMIBC. Among 276 patients over a median follow-up of 22.9 months, 39% of patients were CIS alone, 26% were Ta high grade, 21% were T1 high grade, and 13% were Ta low grade. Overall, 53% had one BCG induction course, and 46% had 2+ BCG induction courses, and 38% were “BCG unresponsive.” Of note, some responders went on to maintenance therapy (monthly versus the SWOG schedule) for 24 months. One and 2-year recurrence-free survival rates were 60% and 46%, and high-grade recurrence-free survival rates were 65% and 52%, respectively. Ten patients (3.6%) had disease progression on transurethral resection, and 43 patients (15.6%) went on to cystectomy (median 11.3 months from induction), of whom 11 (4.0%) had progression to muscle invasion. Importantly, when assessing complete response/high-grade recurrence-free survival at 3 and 12 months for approved and selected treatment options for BCG-unresponsive NMIBC, gemcitabine + docetaxel has impressive and durable responses for both CIS and HG Ta/T1 disease:8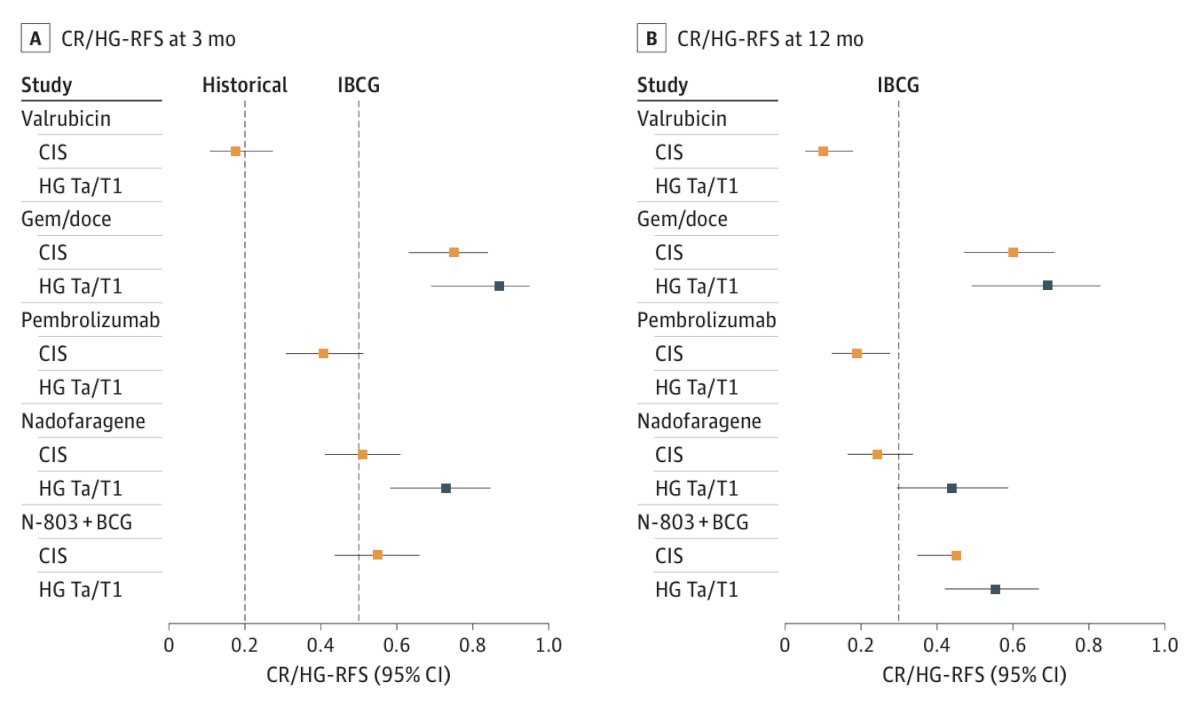
Dr. Kamat’s group has also recently shown that a subpopulation of patients that have BCG-unresponsive CIS +/- Ta/T1 may have a response to additional BCG.9 Among 17 patients receiving additional BCG, 14 (83%) had a complete response: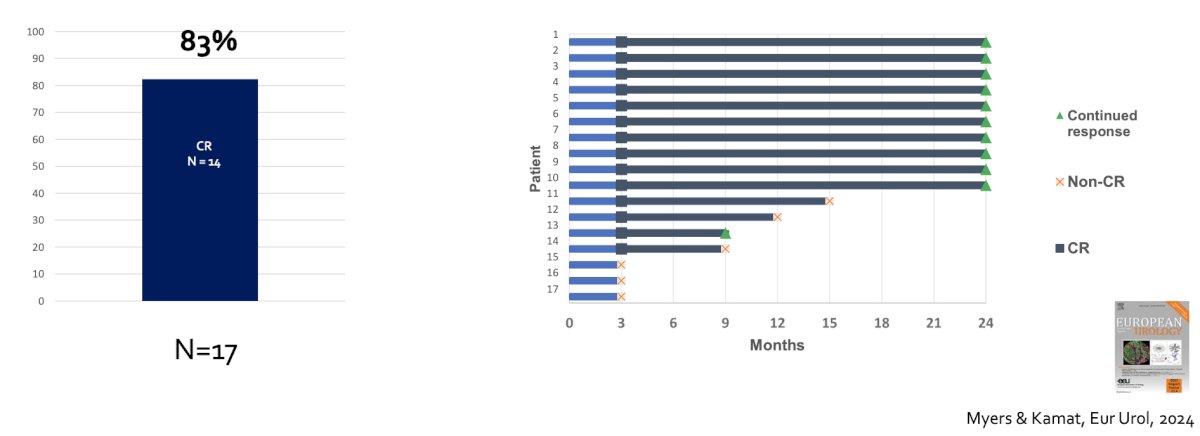
Finally, Dr. Kamat discussed the IBCG’s recommendations for optimal sequencing and patient selection among patients with BCG unresponsive NMIBC.10 For patients with BCG-unresponsive CIS, gemcitabine + docetaxel, nadofaragene firadenovec, and NAI + BCG are recommended; because of its systemic toxicity, pembrolizumab should only be offered after other options are exhausted. For patients with BCG-unresponsive papillary-only tumors, gemcitabine + docetaxel, nadofaragene firadenovec, NAI + BCG, single-agent chemotherapy, hyperthermic mitomycin C, and pembrolizumab are recommended: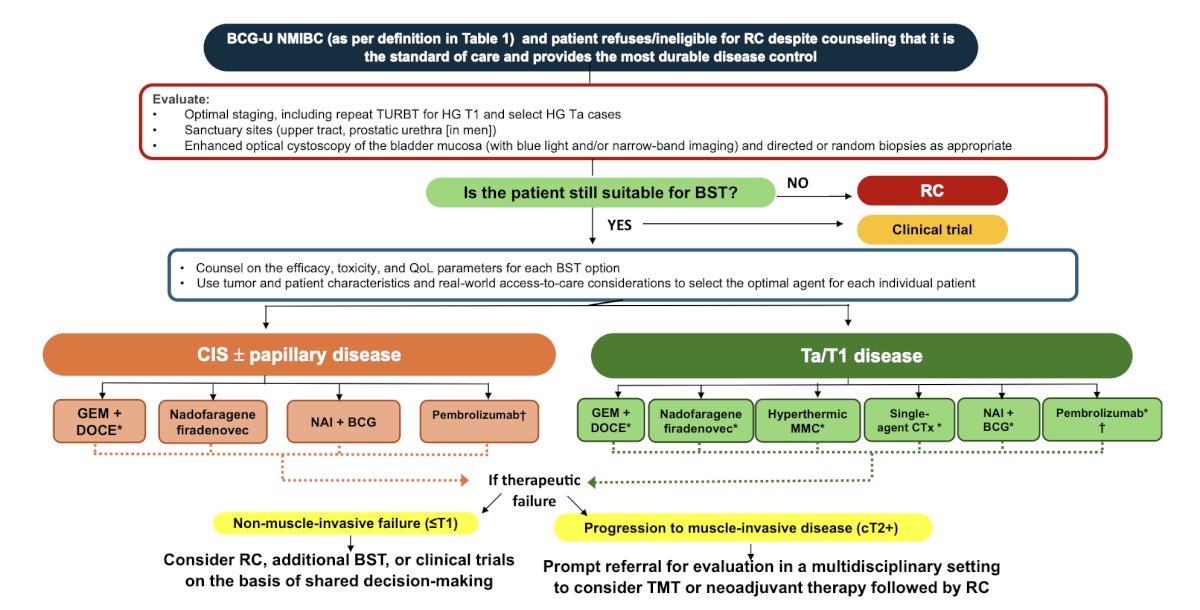
Dr. Kamat concluded his presentation discussing BCG unresponsive cancer in 2024 by highlighting the many questions that remain in this disease space from a patient’s perspective:
- Am I giving up long-term cure for short-term gain?
- Quality of life – is cystectomy better?
- Anxiety – mine? My family?
- Do I still participate in clinical trials?
- How many different therapies can I safely try?
- How do I choose bladder sparing versus upfront cystectomy?
- What are the long-term side effects from drugs?
- Logistical challenges – every week visit versus every 3 months?
- Burden of repeated testing, surveillance?
- Financial toxicity?
Presented by: Ashish Kamat, MD, MBBS, Professor of Urology, and Wayne B. Duddleston Professor of Cancer Research, University of Texas, MD Anderson Cancer Center, Houston, TX
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Society of Urologic Oncology (SUO) Annual Meeting, Dallas, TX, Tues, Dec 3 – Fri, Dec 6, 2024.
Related content: BCG Unresponsive Cancer in 2024 - Ashish Kamat
References:
- Lerner SP, Dinney C, Kamat A, et al. Clarification of Bladder Cancer Disease States Following Treatment of Patients with Intravesical BCG. Bladder Cancer. 2015 Mar 30;1(1):29-30.
- Kamat AM, Sylvester RJ, Bohle A, et al. Definitions, End Points, and Clinical Trial Designs for Non-Muscle-Invasive Bladder Cancer: Recommendations From the International Bladder Cancer Group. J Clin Oncol. 2016 Jun 1;34(16):1935-1944.
- Balar AV, Kamat AM, Kulkarni GS, et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): An open-label, single-arm, multicenter, phase 2 study. Lancet Oncol. 2021 Jul;22(7):919-930.
- Necchi A, Roumiguié M, Kamat AM, et al. Pembrolizumab monotherapy for high-risk non-muscle-invasive bladder cancer without carcinoma in situ and unresponsive to BCG (KEYNOTE-057): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2024 Jun;25(6):720-730.
- Boorjian SA, Alemozaffar M, Konety BR, et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: A single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. 2021 Jan;22(1):107-117.
- Chamie K, Chang SS, Kramolowsky E, et al. IL-15 Superagonist NAI in BCG-Unresponsive Non-Muscle-Invasive Bladder Cancer. NEJM Evid 2022; 2(1).
- Steinberg RL, Thomas LJ, Brooks N, et al. Multi-institution evaluation of sequential gemcitabine and docetaxel as rescue therapy for nonmuscle invasive bladder cancer. J Urol. 2020;203:902-909.
- Hwang TJ, Davies BJ, Preston MA. Advancing Patient-Centered Outcomes and Equity in Clinical Trials for BCG-Unresponsive Nonmuscle Invasive Bladder Cancer. JAMA Oncol. 2023 Nov 1;9(11):1491-1492.
- Myers AA, Tan WS, Grajales V, et al. Challenging the paradigm of “BCG-unresponsive” Bladder Cancer: Does Additional Bacillus Calmette-Guerin have an effect? Eur Urol. 2024 Oct;86(4):366-368.
- Li R, Hensley PJ, Gupta S, et al. Bladder-sparing therapy for Bacillus Calmette-Guerin-unresponsive non-muscle-invasive Bladder Cancer: International Bladder Cancer Group Recommendations for Optimal Sequencing and Patient Selection. Eur Urol. 2024 Aug 24;S0302-2838(24)02516-8.


