(UroToday.com) The 2024 SUO annual meeting included a session on muscle invasive bladder cancer, featuring a presentation by Dr. Jacqueline Brown discussing emerging perioperative systemic therapy. Currently, Dr. Brown notes that when we see a new patient with muscle invasive urothelial carcinoma that is fit for neoadjuvant chemotherapy, the treatment paradigm is for dose dense MVAC for 4-6 cycles or gemcitabine + cisplatin for 3-4 cycles followed by radical cystectomy. If they have high risk disease remaining in their bladder or lymph nodes at the time of radical cystectomy, we then consider treatment with adjuvant nivolumab, and for those who are not a candidate for radical cystectomy or for those that refuse surgery, we often consider trimodality therapy with maximal TURBT followed by chemoradiation:
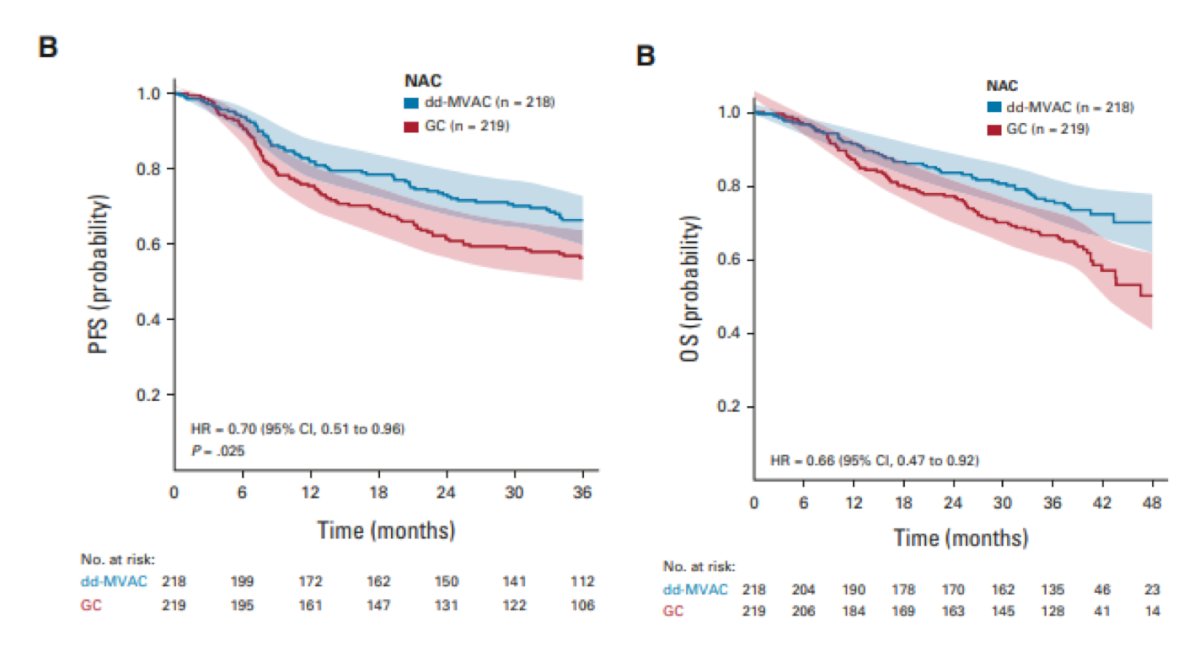
Dr. Brown then discussed the VESPER trial, which attempted to answer the question of what type of chemotherapy to use in the neoadjuvant setting.1 Patients with pT2 disease were randomized to 4 cycles of gemcitabine + cisplatin versus 6 cycles of dose dense MVAC, with 89% of patients receiving treatment in the neoadjuvant setting. Notably, dose dense MVAC improved progression free survival and overall survival compared to gemcitabine + cisplatin with a hazard ratio of 0.70 and 0.66, respectively:
The pathological complete response rate for dose dense MVAC was 42% and for gemcitabine + cisplatin was 36%. However, Dr. Brown notes that the average person in this trial was 62 years of age, nearly a decade younger than our patients in the real world setting. The increased efficacy with dose dense MVAC came at the cost of increased toxicity, specifically febrile neutropenia, fatigue, and gastrointestinal symptoms.
Since 2021, adjuvant nivolumab has been the standard of care based on the CheckMate 274 trial.2 In this phase 3 trial, patients with high risk disease were randomized to one year of adjuvant nivolumab versus placebo. The co-primary endpoints were disease free survival in the intention to treat population and in the PD-L1 positive population:
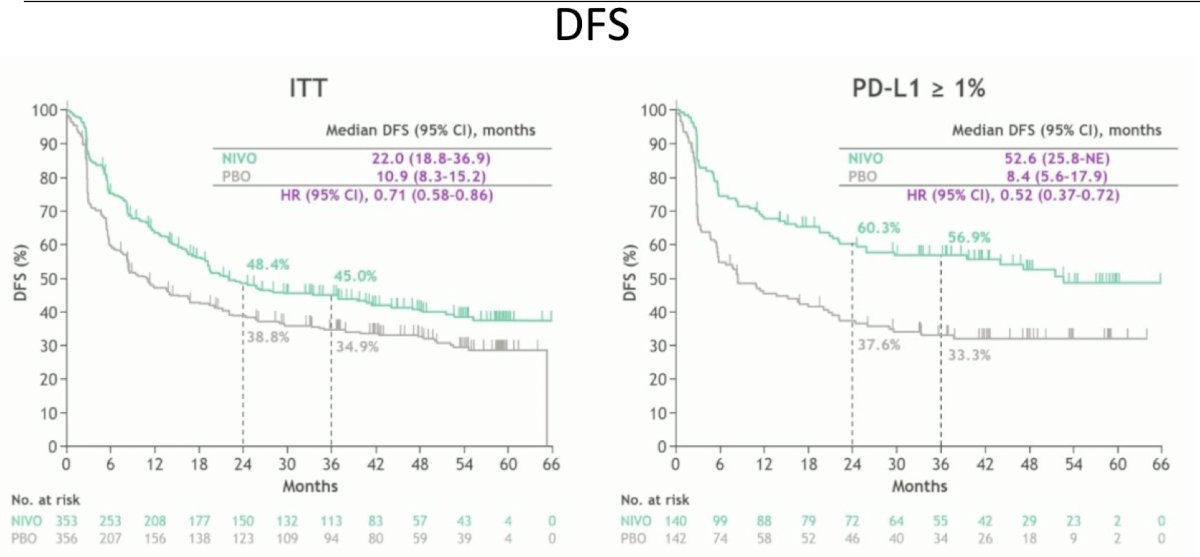
The first analysis of CheckMate-274 noted an improvement in disease free survival among all patients of 22.0 months for nivolumab and 10.9 months for placebo (HR 0.71, 95% CI 0.58-0.86):
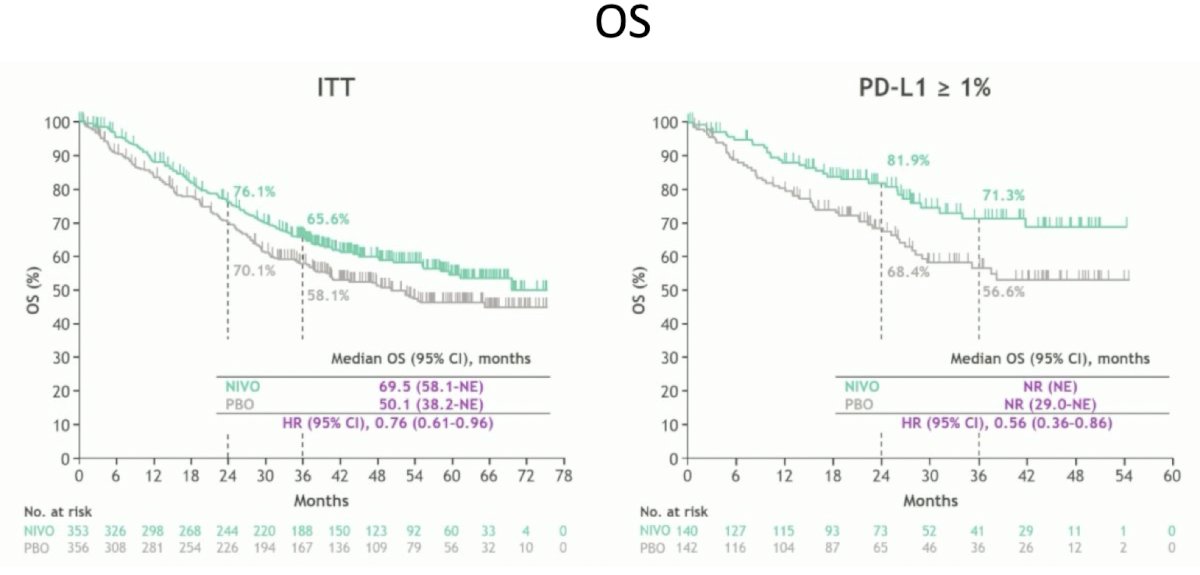
In 2024, Dr. Matt Galsky presented updated results at the EAU annual meeting demonstrating an improvement in overall survival in those who received nivolumab, thus this was the first trial of adjuvant immunotherapy that showed an improvement in overall survival:
Subsequently, Dr. Brown discussed the evolving landscape of perioperative systemic therapy. There are three goals for patients with muscle invasive urothelial carcinoma:
- Avoid overtreatment of patients who can be cured with fewer modalities
- Avoid under treatment in patients who can be cured with escalation of therapy
- Avoid cystectomy in those with incurable micrometastatic disease
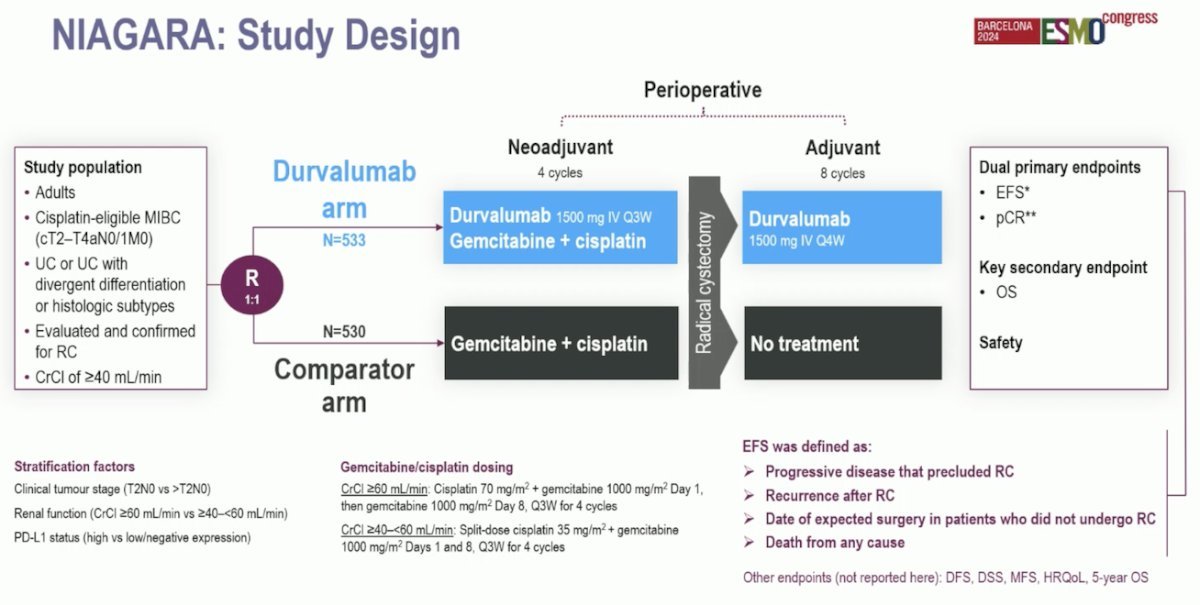
Dr. Brown notes that there are a few concepts to keep in mind, specifically that some of these studies are neoadjuvant studies, some are adjuvant studies, and some include a perioperative approach with both a neoadjuvant and an adjuvant component. Additionally, many of these trials are attempting to refine biomarkers to better determine who needs treatment and who is likely to respond to treatment. The NIAGARA trial was presented at ESMO 2024, which was a phase 3 study of patients with T2-4a or N0-1 disease randomized to neoadjuvant gemcitabine + cisplatin with or without the anti-PD-L1 checkpoint inhibitor durvalumab. The dual primary endpoints were event free survival and pathological complete response rate:
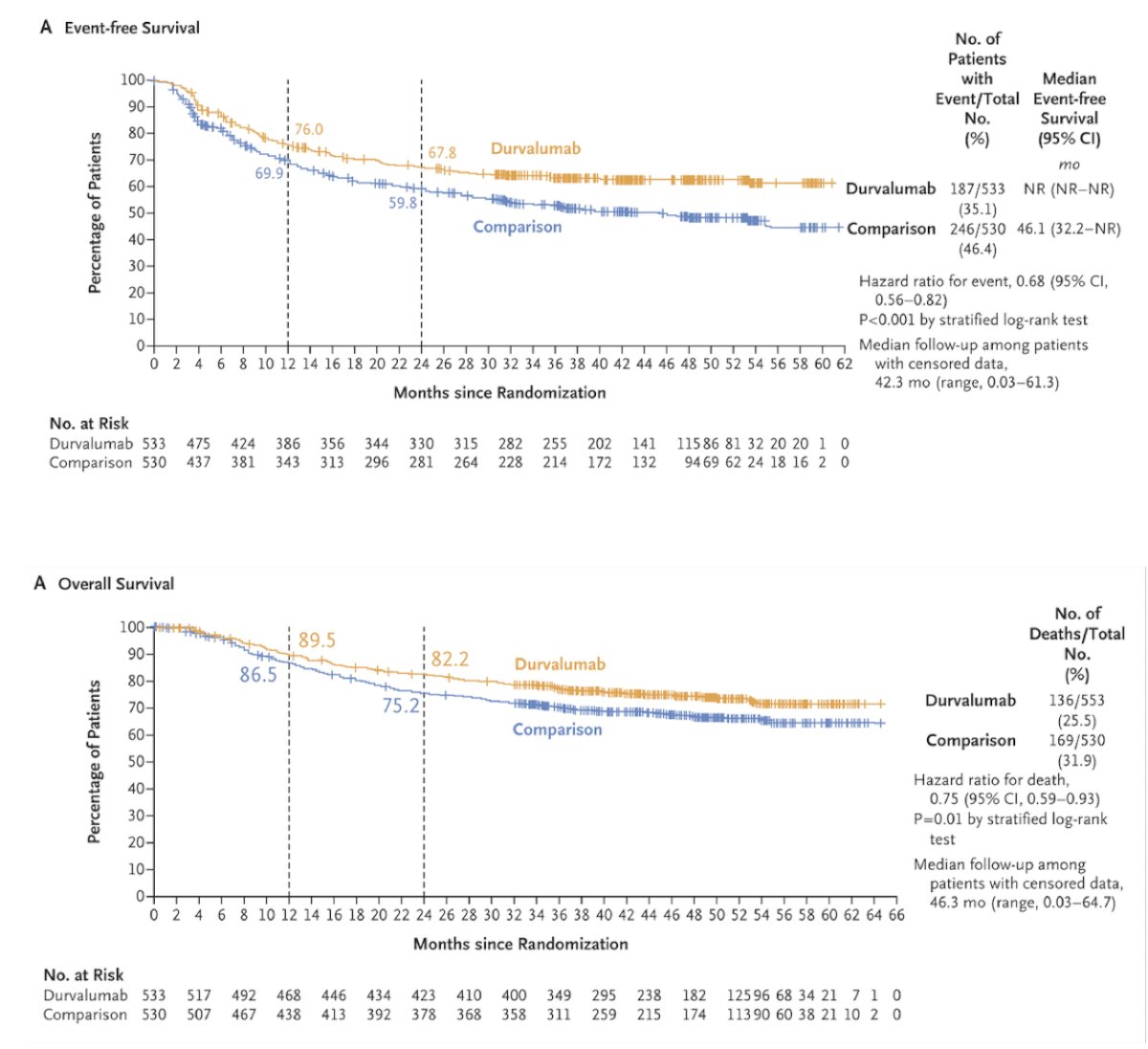
NIAGARA showed that the addition of durvalumab to gemcitabine + cisplatin resulted in an improved event free survival (HR 0.68, 95% CI 0.56-0.82), as well as an improvement in overall survival (HR 0.75, 95% CI 0.59-0.93):
Moreover, the pathologic complete response rate favored the addition of durvalumab: 37.3% versus 27.5% (RR 1.34, 95% CI 1.13-1.60). Dr. Brown emphasized that this trial did an adequate job of recruiting a more “real-world population” including patients with a CrCl >= 40 rather than CrCl >= 60 (allowing split dose cisplatin), as well as including patients with N1 disease. However, all patients randomized to the durvalumab arm received both neoadjuvant and adjuvant treatment, which includes patients who had a significant downstage or pathologic complete response with the neoadjuvant component, raising the question about potential overtreatment of these patients.
Additionally, how we compare this data to Checkmate-274 and adjuvant nivolumab after cisplatin based chemotherapy remains to be established.
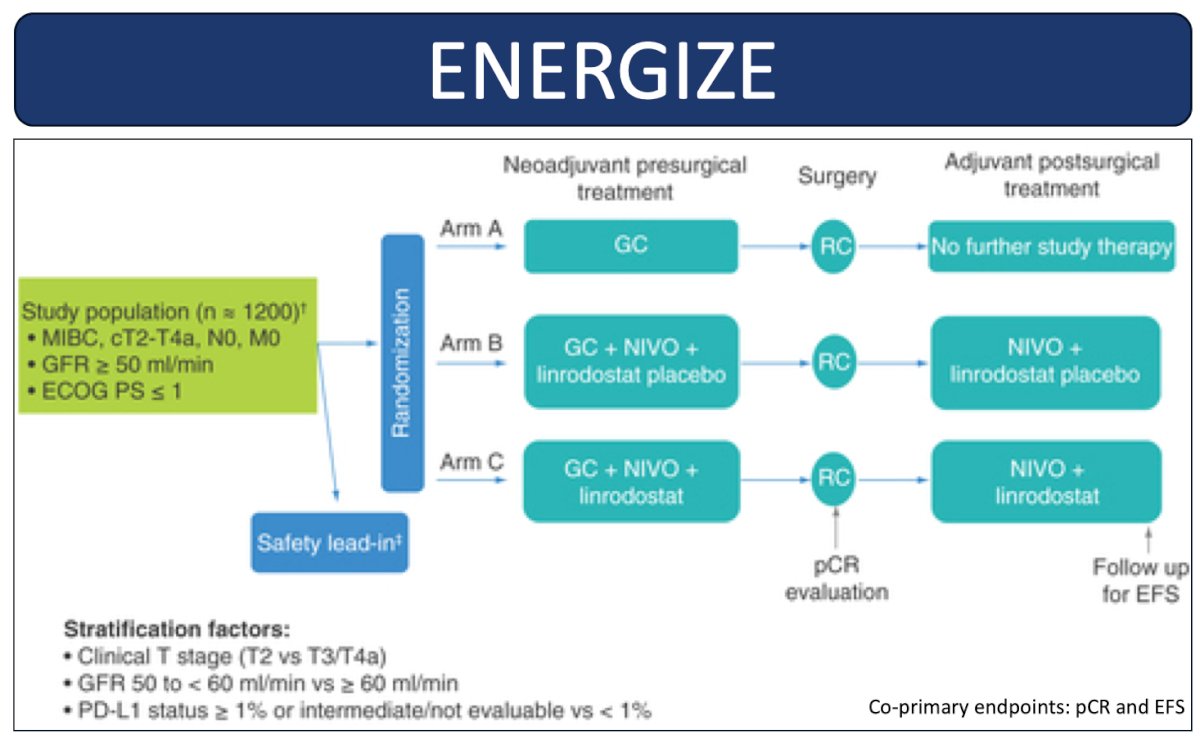
Dr. Brown notes that there are two other ongoing phase 3 trials of perioperative chemotherapy with or without immunotherapy. ENERGIZE is randomizing N0 muscle invasive bladder cancer patients to either neoadjuvant chemotherapy alone, neoadjuvant chemotherapy with perioperative nivolumab, or a third arm assessing intensification of immunotherapy with perioperative gemcitabine + cisplatin, nivolumab, and linrodostat:
KEYNOTE 866 is looking at gemcitabine + cisplatin with or without perioperative pembrolizumab:
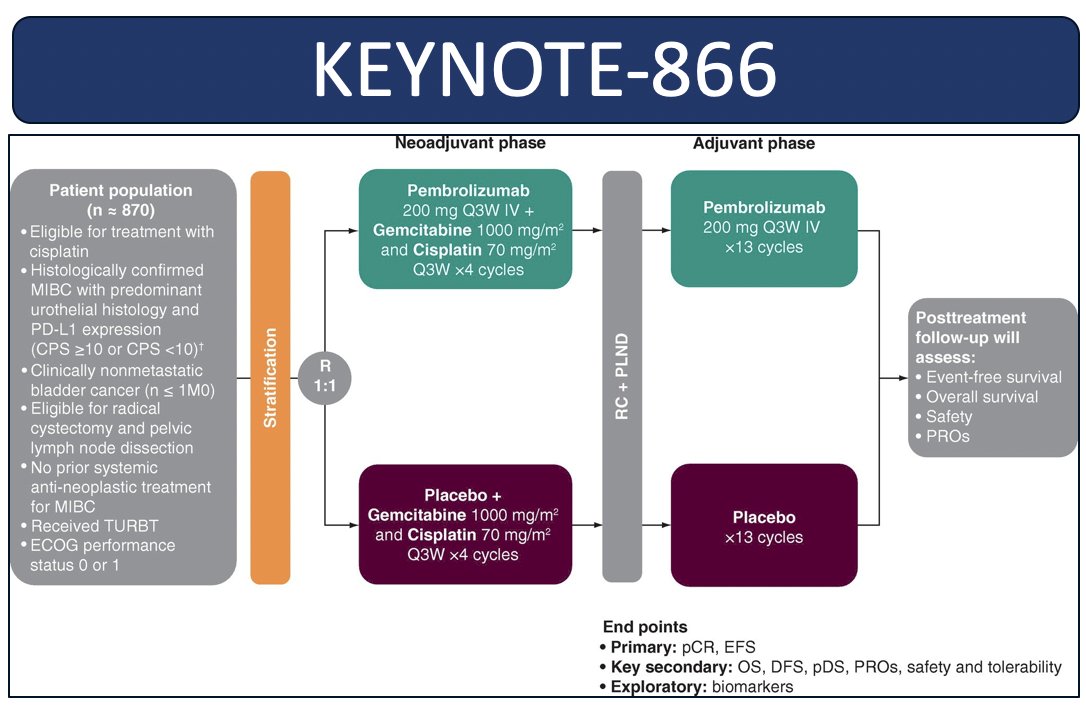
The co-primary endpoints for both of these trials are pathologic complete response and event free survival and will be very important to understanding the role of perioperative chemo-immunotherapy in muscle invasive bladder cancer patients. As follows is a brief summary of adjuvant immunotherapy monotherapy phase 3 trials, highlighting CheckMate-274, AMBASSADOR,4 and IMvigor0105:
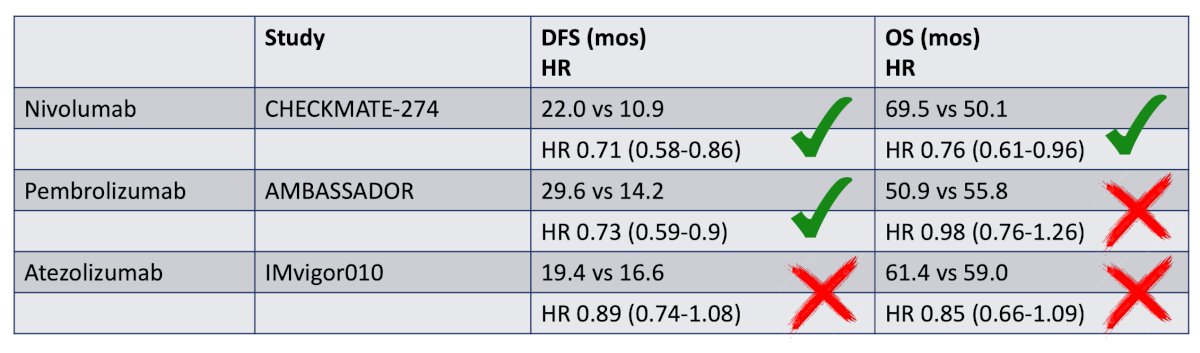
Dr. Brown emphasized that what these trials collectively show is that we need better tools to assess which patients actually need adjuvant therapy. As such, a post hoc analysis of IMvigor010 showed that overall survival was improved in patients who were ctDNA positive at the time of starting treatment and received atezolizumab (HR 0.59, 95% CI 0.42-0.83). However, those with ctDNA-negative status had similar overall survival between arms and seemingly benefitted less from adjuvant atezolizumab (HR 1.38, 95% CI 0.93-2.05):
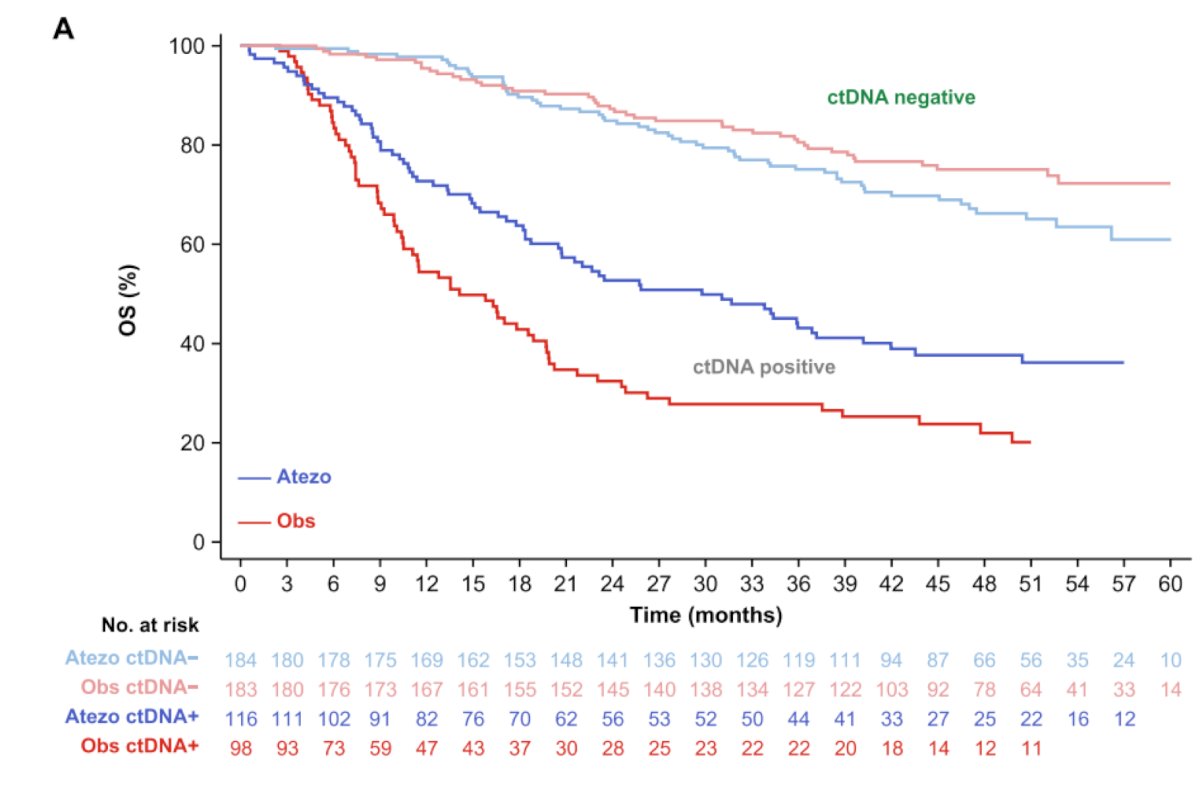
Thus, ctDNA positive status may be a tool that could help identify patients with muscle invasive urothelial carcinoma who might benefit from adjuvant immunotherapy.
Next, Dr. Brown discussed novel agents making an impact in the perioperative space – all of which are antibody drug conjugates. For context, she noted that advanced/metastatic urothelial carcinoma has experienced a paradigm shift in the last year with the approval of enfortumab vedotin + pembrolizumab in both cisplatin eligible and cisplatin ineligible patients. This combination resulted in a landmark 31.5 month median overall survival compared to 16.1 months for chemotherapy6:
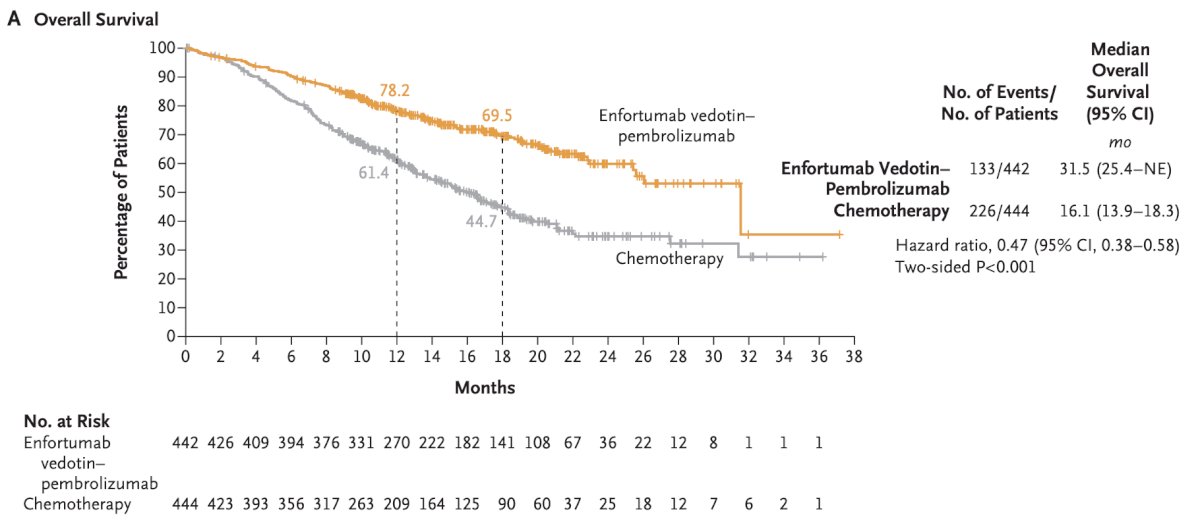
Additionally, the complete response rate was 29.1% and the objective response rate was 67.7%.
So, what about enfortumab vedotin in the perioperative space? Two phase 2 trials of perioperative enfortumab vedotin include EV-103 cohort H and cohort L. In cohort H, patients with muscle invasive bladder cancer received 3 cycles of neoadjuvant enfortumab vedotin prior to radical cystectomy in cisplatin ineligible patients, of which 22 patients were enrolled:
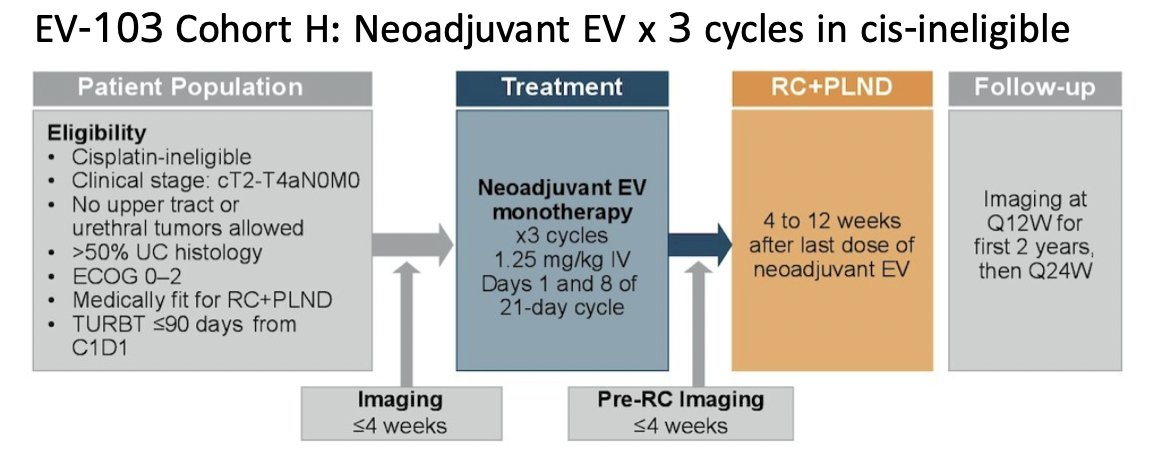
There was a 36.4% pathologic complete response rate and 50% rate of downstaging to less than muscle invasive bladder cancer. Overall, 86% of patients completed all 3 cycles and 100% proceeded to radical cystectomy. The 24 month event free survival was 62%. Cohort L assessed perioperative enfortumab vedotin in cisplatin ineligible patients, whereby patients received 3 cycles of enfortumab vedotin prior to radical cystectomy followed by 6 cycles post-operatively:
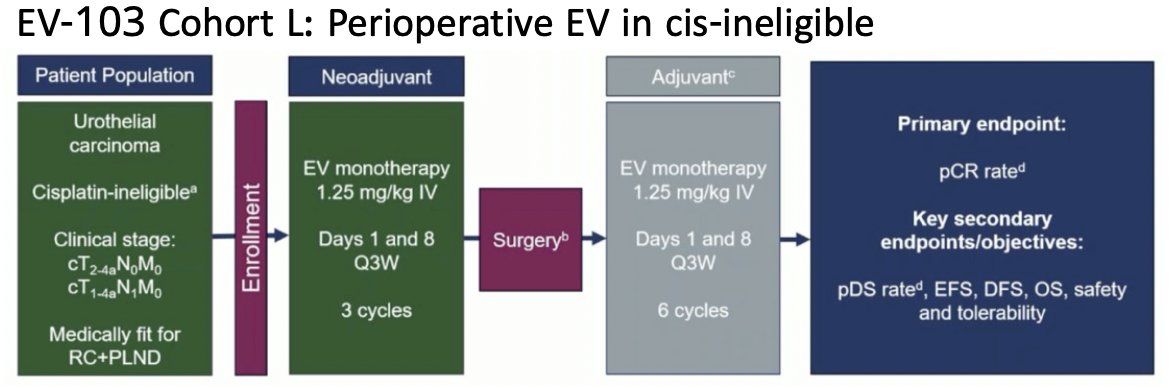
There were 51 patients enrolled, with a pathological complete response rate of 34% and a pathologic downstaging rate of 42%. Overall, 82.4% completed 3 cycles of neoadjuvant therapy and proceeded to radical cystectomy, and enfortumab vedotin related toxicities did not prevent any patients from proceeding to radical cystectomy. Building on these trials are EV-303 and EV-304. EV-303 is a trial in which cisplatin ineligible patients with muscle invasive bladder cancer (up to N1) are randomized to perioperative pembrolizumab versus observation versus perioperative pembrolizumab + enfortumab vedotin, 3 cycles prior and 6 cycles after radical cystectomy. Event free survival is primary endpoint:
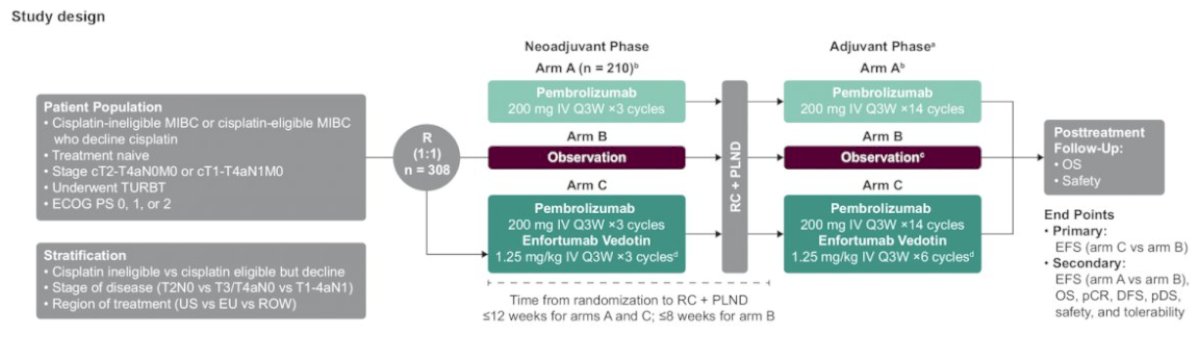
EV 304 is a study of perioperative enfortumab vedotin + pembrolizumab in patients who are cisplatin eligible with a comparator arm of gemcitabine + cisplatin:
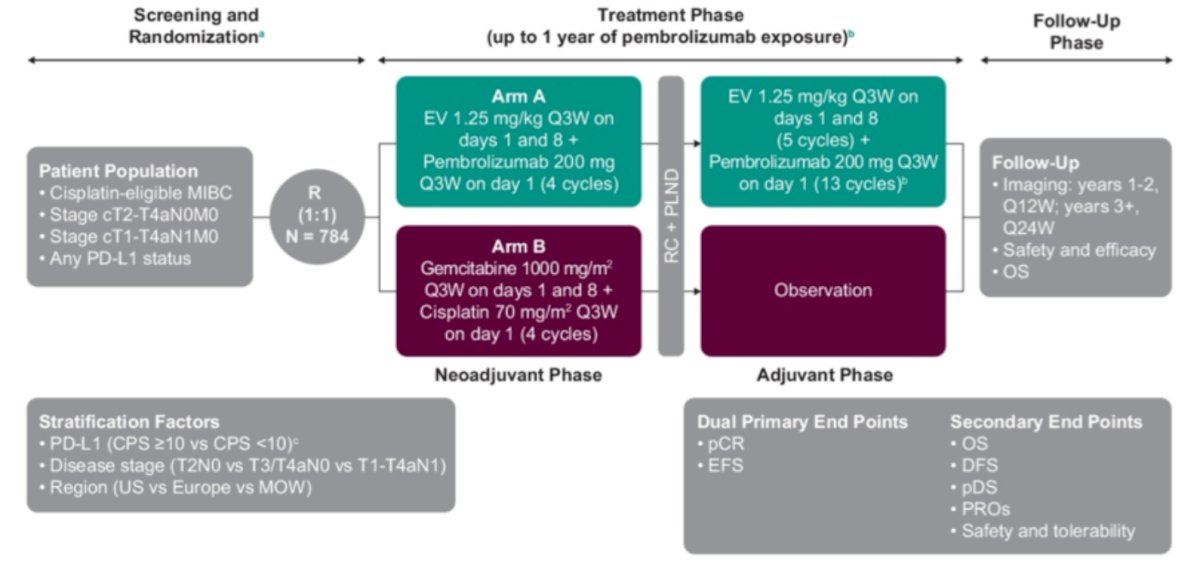
Both of these trials have completed accrual and we are awaiting results.
The VOLGA trial is a phase 3 trial of perioperative enfortumab vedotin with or without intensified immunotherapy in cisplatin ineligible patients. Patients are randomized in a 1:1:1 fashion to receive radical cystectomy alone, enfortumab vedotin + durvalumab, or enfortumab vedotin + durvalumab with a boost from the anti CTLA4 inhibitor tremelimumab:
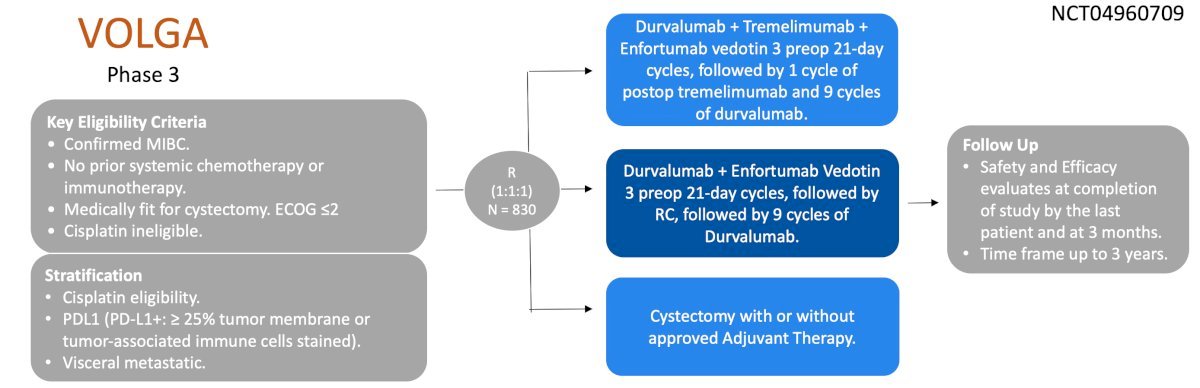
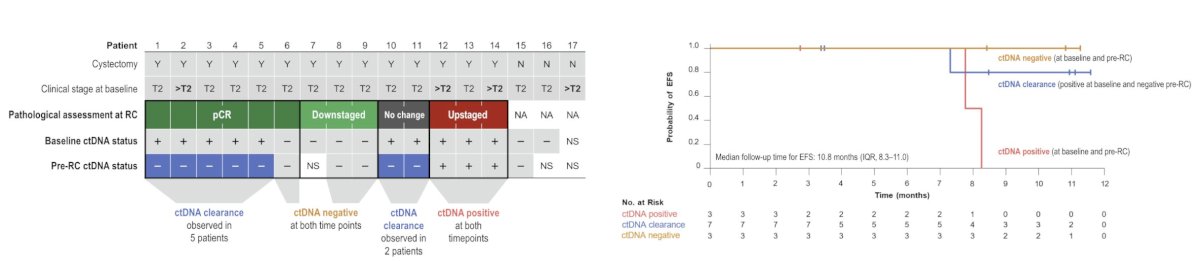
At EMSO 2024, Dr. Alex Drakaki presented an exploratory analysis of plasma ctDNA from the safety run-in of VOLGA. Pathologic complete response was seen in 6/17 patients and pathologic non-muscle invasive response was seen in 9/17 patients. Among 6 patients with pathologic complete response, ctDNA clearance was observed in 5 patients, and among 3 downstaged patients, ctDNA was negative at both time points. In the patients who were upstaged, ctDNA positivity persisted in all three patients between these two time points. Longer event free survival was observed in the ctDNA clearance and ctDNA negative groups compared with the ctDNA positive group:
Sacituzumab govitecan is another antibody drug conjugate that in the phase 2 TROPHY-U-01 cohort 1 showed (as monotherapy in metastatic urothelial carcinoma after platinum chemotherapy and immunotherapy) an objective response rate of 27%. However, the phase 3 TROPiCS-04 trial of sacituzumab govitecan versus third line chemotherapy (based on a May 30, 2024 press release) did not meet its primary endpoint of overall survival and also led to an excess of neutropenic deaths. In the perioperative setting of the SURE-01 trial (n = 21), sacituzumab govitecan led to 75% of the first eight patients having grade 3/4 neutropenia, leading to a protocol amendment of dose reduction and treatment with empiric G-CSF. Overall, 11 of 18 patients proceeded to radical cystectomy. Dr. Brown notes that this trial highlights that the fourth goal in treating muscle invasive bladder cancer with neoadjuvant therapy is not preventing anyone from receiving a radical cystectomy.
The anti-HER2 antibody drug conjugate disitamab vedotin has been shown in the metastatic setting as a monotherapy refractory to platinum chemotherapy to have an objective response rate of 50% in HER 2/3+ patients. In combination with a PD-1 inhibitor in the treatment naïve setting, disitamab vedotin has been associated with an objective response rate of 76%, and up to 83.3% in HER2 IHC 2/3+ patients (and only 33% in IHC 0 patients). In the neoadjuvant setting, a phase 2 trial of 12 patients treated with 3 cycles of disitamab vedotin + toripalimab reported at an interim analysis to have a pathologic complete response rate of 80% (8/10 patients), with only one treatment related adverse event and no grade 4 events.
Finally, Dr. Brown discussed TAR-200, which is an intravesical drug delivery system that provides local continuous gemcitabine release within the bladder. SunRISe-4 is the phase 2 study of neoadjuvant TAR-200 + systemic cetrelimab versus neoadjuvant cetrelimab alone in patients with muscle invasive bladder cancer planned for radical cystectomy and are cisplatin ineligible. The primary endpoint is pathologic complete response rate at radical cystectomy:
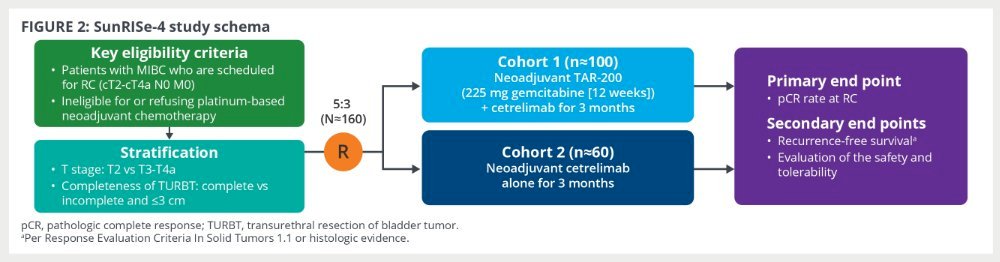
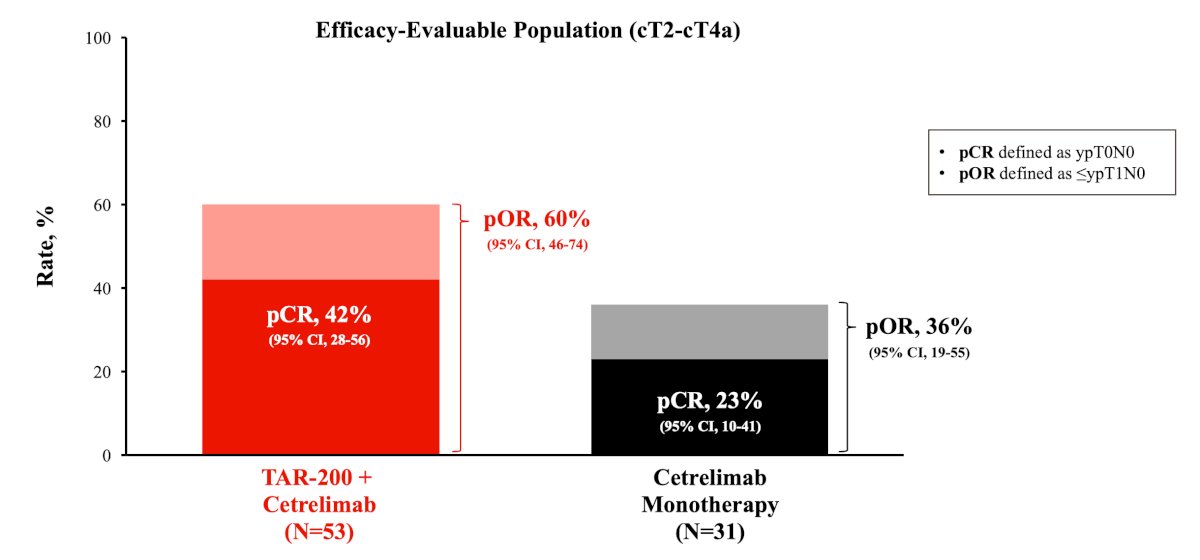
At ESMO 2024, Dr. Andrea Necchi presented the results of an interim analysis of SunRISe-4, noting a pathological complete response rate in the combination arm of 42% and pathologic objective response rate of 60%. The corresponding proportions in the cetrelimab monotherapy arm were 23% and 36%, respectively:
Notably, the adverse events from TAR-200 were primarily local, although systemic exposure with a checkpoint inhibitor led to systemic immune related adverse events. However, only 13% of patients discontinued treatment due to treatment related adverse events.
Dr. Brown concluded her presentation by discussing emerging perioperative systemic therapy with the following take home messages:
- NIAGARA is the most recent potentially practice-changing data supporting perioperative chemo-immunotherapy, but questions remain
- ctDNA is a promising tool under investigation for optimizing adjuvant immunotherapy
- Enfortumab vedotin has been practice-changing in advanced disease and perioperative combination studies are anxiously awaited
- Agents like disitamab vedotin, sacituzumab govitecan, and TAR-200 have shown efficacy
- The neoadjuvant setting is ideal for evaluation of novel therapies, but the burden of safety is intensified given curative intent
Presented by: Jacqueline T. Brown, MD, Emory University, Atlanta, GA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Society of Urologic Oncology (SUO) Annual Meeting, Dallas, TX, Tues, Dec 3 – Fri, Dec 6, 2024.
References:
- Pfister C, Gravis G, Flechon A, et al. Dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin or gemcitabine and cisplatin as perioperative chemotherapy for patients with nonmetastatic muscle-invasive bladder cancer: Results of the GETUG-AFU V05 VESPER trial. J Clin Oncol. 2022 Jun 20;40(18):2013-2022.
- Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021 Jun 3;384(22):2102-2114.
- Powles T, Catto JWF, Galsky MD, et al. Perioperative Durvalumab with Neoadjuvant Chemotherapy in Operable Bladder Cancer. N Engl J Med. 2024 Nov 14;391(1):1773-1786.
- Apolo AB, Ballman KV, Sonpavde G, et al. Adjuvant pembrolizumab versus observation in muscle-invasive urothelial carcinoma. N Engl J Med. 2024 Sep 15 [Epub ahead of print].
- Bellmunt J, Hussain M, Gschwend JE, et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): A multicentre, open-label, randomized, phase 3 trial. Lancet Oncol. 2021 Apr;22(4):525-537.
- Powles T, Valderrama BP, Gupta S, et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N Engl J Med. 2024 Mar 7;390(10)875-888.


