(UroToday.com) The 2024 SUO annual meeting included a session on non muscle invasive bladder cancer (NMIBC), featuring a presentation by Dr. Sima Porten discussing the heterogeneity of intermediate risk NMIBC. Based on the AUA guidelines, low risk NMIBC has a <5% risk of progression and a ~45% risk of recurrence, whereas high risk disease has a ~75% risk of recurrence and 30% risk of progression. However, intermediate risk disease represents a mixed bag of clinical disease states, with heterogeneity within the guidelines:

The definition of intermediate risk NMIBC stratified by guidelines is as follows:
- AUA
- Ta LG, recurrence < 1 year
- Single Ta LG, > 3 cm
- Ta LG, multifocal
- Ta HG < 3 cm
- T1 LG
- EAU
- Ta LG/G1 or Ta LG/G2 with two or less risk factors (patient less than 70 years of age, < 3 cm, multiple tumors)
- Ta HG/G3 or T1 LG with only one risk factor
- IBCG (where all high grade tumors are high risk)
- Ta LG (recurrence, 1 year, > 3 cm, multifocal)
According to Dr. Porten, work done by Dr. Ashish Kamat’s group suggests that all high-grade Ta tumors should be classified as high-risk based on evidence of their response to BCG.1 Among patients treated at the MD Anderson Cancer Center, BCG unresponsiveness developed in 13% of high-risk high-grade Ta tumors and 14% of intermediate-risk high-grade Ta tumors compared to 0% of intermediate-risk low grade Ta tumors (p = 0.003). While no patients with intermediate-risk low grade Ta tumors progressed, progression rates were similar in high-risk high-grade Ta tumors and intermediate-risk high-grade Ta tumors (≥T2: 5.9% and 6.5%; T1: 13% and 13%, respectively).
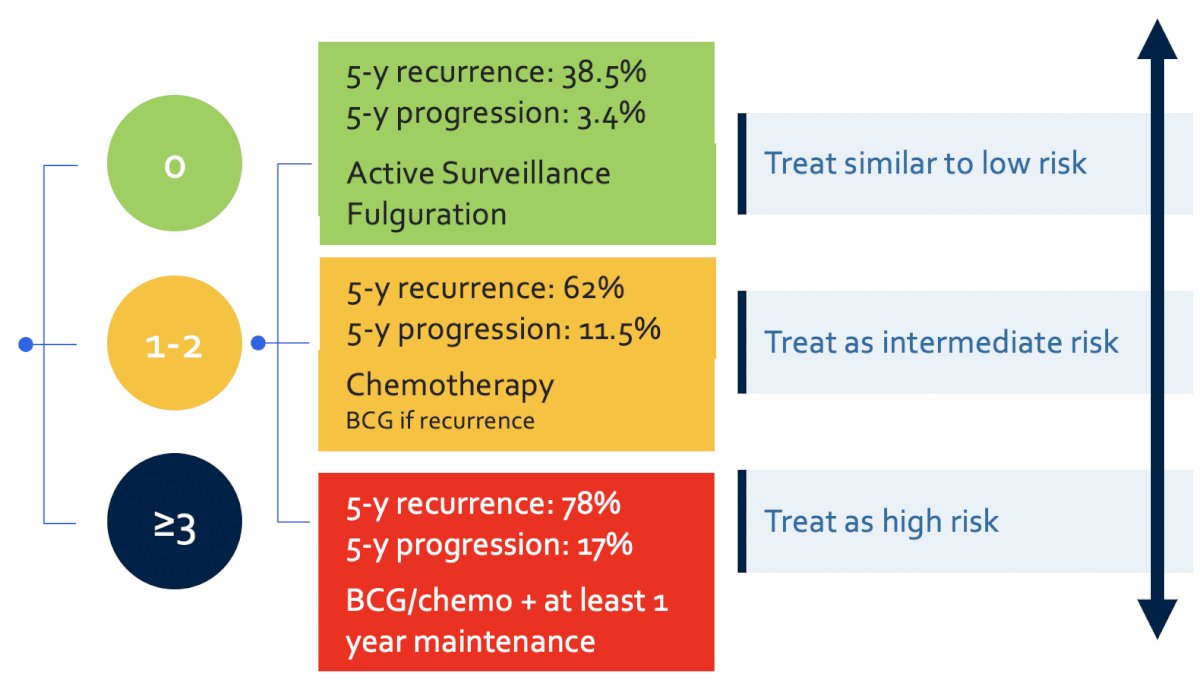
Work from the International Bladder Cancer Group has provided updated definitions and management recommendations for the treatment of intermediate risk non-muscle invasive bladder cancer.2 Among intermediate risk (low-grade tumors), the following risk factors should be assessed:
- Tumor size >3 cm
- Multiple tumors
- Early recurrence (<1 year)
- Frequent recurrence (>1/year)
- Failure of previous intravesical treatment
For those patients with none of the above risk factors, they should be treated similarly to those with low-risk disease (no additional treatment). For patients with 1-2 risk factors, should be treated as intermediate risk disease and thus treated with additional adjuvant induction intravesical chemotherapy (or BCG if prior chemotherapy has been used). For patients with >= 3 risk factors, they should be treated as high risk disease with BCG/chemotherapy for at least 1 year, with maintenance therapy:
Dr. Porten emphasized that individual patient considerations also lead to heterogeneity. This includes age (fertility considerations, cognitive decline), comorbidities (bladder function, frailty, anticoagulation), financial concerns (caregiver responsibilities, insurance vs out of pocket expenses, time off work), and social determinants of health (access, transportation). TURBT is also associated with side effects in this population:
- 30 day complications: 5.1%
- Transfusion: 1.5%
- Readmission: 3.7% (29% from bleeding, 21% from infection)
- Reoperation: 1.5%
- Mortality: 0.8%
- Post-operative delirium: 65%
- Anesthesia related long term cognitive decline: 10%
Dr. Porten noted that a 2024 AUA Update series highlighted the current literature and suggests that patients like active surveillance (compliance is excellent), it is feasible (median time on active surveillance: 9-95 months, re-TURBT was avoided in up to 70% of cases), and it was effective/safe (grade progression < 20%, stage progression < 13%, and progression to muscle invasive bladder cancer was only seen in high grade pT1):
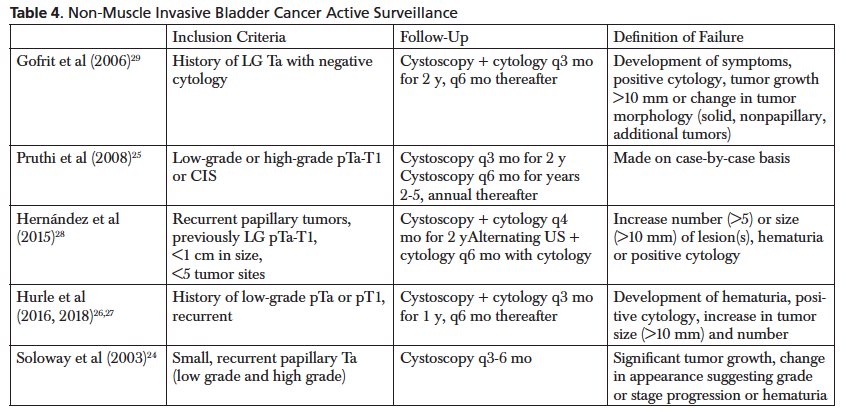
Ultimately, patient selection is key and this should be used for only low grade recurrence. The use of cytology helps to rule out a high grade recurrence.
Office fulguration is also feasible, and effective, and patients like it. There is a low risk of reclassification to high risk non muscle invasive bladder cancer (~5-20%), the accuracy of predicting low grade disease is 85-93%, for 1 cm tumors, the 2 year recurrence free survival is similar to TURBT (28% vs 26%), VAS of 0-3 (no, mild pain) is noted in 86% of patients, with 97% of patients preferring office fulguration under local anesthesia rather than going to the operating room.
Furthermore, there may be additional molecular heterogeneity involved in intermediate risk NMIBC. FGFR (activating) alterations are frequent (50%-80%) and common in LG Ta tumors. As such, oral agents are toxic, thus novel delivery systems providing sustained drugs with low side effects may be a useful treatment strategy across clinical heterogeneity of intermediate risk NMIBC. TAR-210 is designed for sustained intravesical delivery of erdafitinib over 90 days while limiting systemic toxicities. Among 30 patients with intermediate risk NMIBC, recurrent, low grade only Ta/T1 disease, 28 patients (93%) had a complete response and 86% (24/28) of complete responses were ongoing at the time of clinical cutoff. Estimated median duration of response was 12.2 months (95% CI 8.3-NE).
Dr. Porten concluded her presentation by discussing the heterogeneity of intermediate risk NMIBC with the following take home messages:
- There is heterogeneity in every aspect of the current clinical paradigm for intermediate risk NMIBC, even if you take out HG Ta from this bucket
- There is a solid framework and set of tools with the sub-stratification system outlined by IBCG, which has been validated and can help guide success rate of strategies (like active surveillance compared with adjuvant therapy)
- We should counsel patients using this framework and choose appropriate tools within the context of individual patient heterogeneity
- Future directions will exploit molecular similarity and utilize novel delivery systems to increase our ability to reduce recurrence (eliminate progression) without sacrificing quality of life
Presented by: Sima Porten, MD, MPH, University of California – San Francisco, San Francisco, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Society of Urologic Oncology (SUO) Annual Meeting, Dallas, TX, Tues, Dec 3 – Fri, Dec 6, 2024.
References:
- Bree KK, Hensley PJ, Lobo N, et al. All High-Grade Ta Tumors Should be Classified as High Risk: Bacillus Calmette-Guerin Response in High-Grade Ta Tumors. J Urol. 2022 Aug;208(2):284-291.
- Tan WS, Steinberg G, Witjes JA, et al. Intermediate-risk non-muscle invasive bladder cancer: Updated Consensus Definition and Management Recommendations from the International Bladder Cancer Group. Eur Urol Onc. 2022 Oct;5(5):505-516.


