(UroToday.com) The 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas was host to the Bladder Cancer Course and the session Metastatic Urothelial Cancer. Dr. Vadim S. Koshkin discussed Emerging Treatments and New Targets for Metastatic Urothelial Cancer.
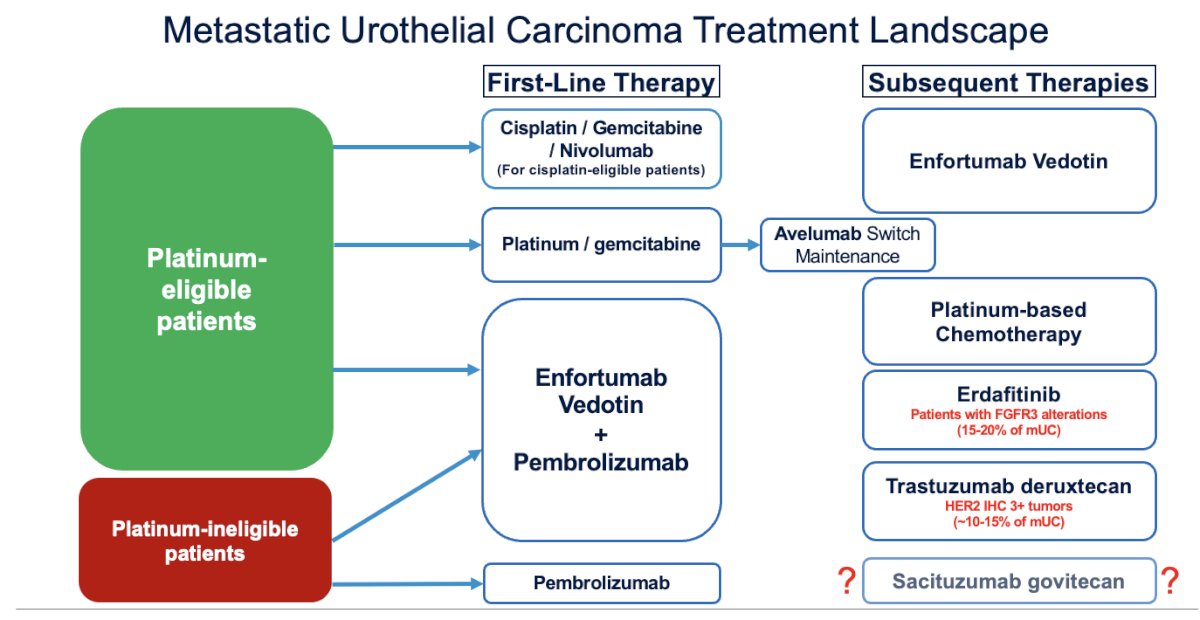
Dr. Koshkin began by highlighting the current treatment landscape for metastatic urothelial carcinoma. Most patients will receive enfortumab vedotin (EV) + pembrolizumab as first-line treatment; however, in some cases, patients may be treated with a cisplatin-based regimen followed by maintenance with avelumab
.
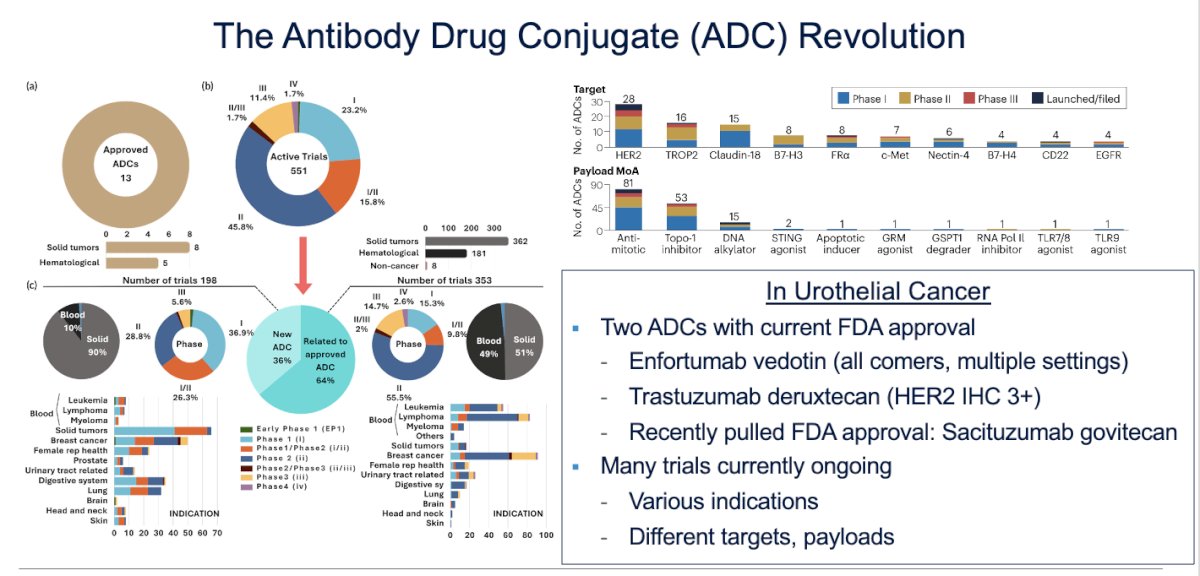
Dr Koshkin emphasized the significant progress made in the treatment of metastatic urothelial carcinoma, particularly over the past 5 to 7 years. The treatment landscape has changed dramatically, with the antibody-drug conjugate (ADC) revolution extending beyond urothelial carcinoma to oncology as a whole, with over 551 active trials. Specifically, in urothelial carcinoma, two drugs have been approved: trastuzumab deruxtecan, an anti-HER2 antibody, and enfortumab vedotin (EV), an anti-Nectin-4 antibody.
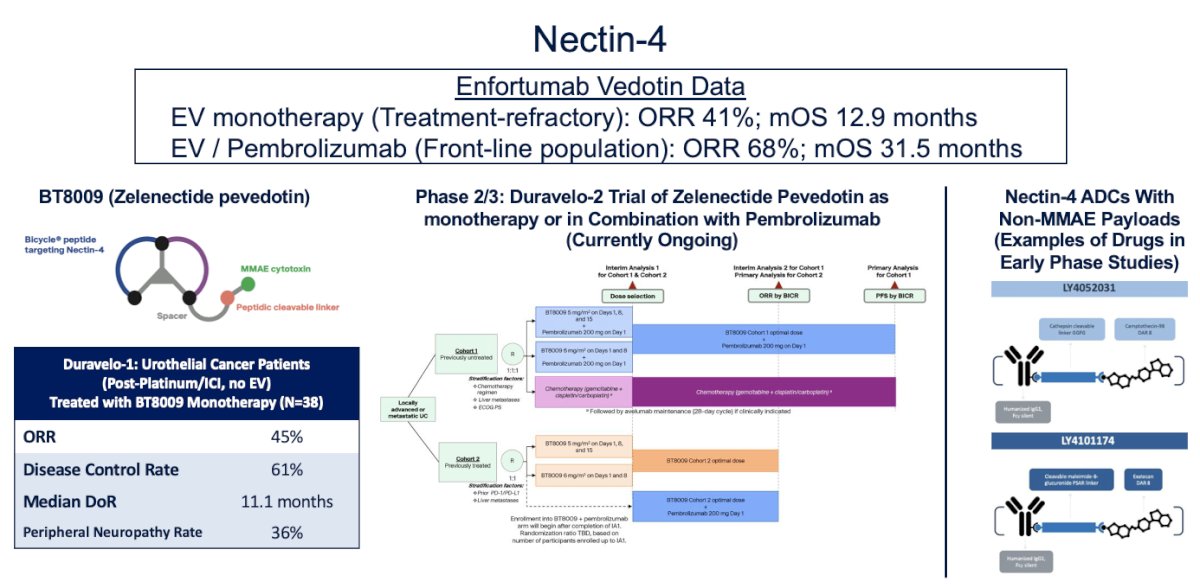
Nectin-4 has proven to be one of the most successful targets in urothelial carcinoma to date. EV as monotherapy, demonstrated an impressive overall response rate (ORR) of 41% and a median overall survival (OS) of 12.9 months in the refractory setting. When combined with pembrolizumab, EV showed an ORR of 68% and a median OS of 31.5 months in the first-line setting of metastatic urothelial carcinoma, doubling the OS previously observed with chemotherapy.
Notably, enfortumab vedotin (EV) is not the only compound targeting Nectin-4. Zelenectide pevedotin, which is designed to resemble a bike, is a peptide that targets Nectin-4 but is not an ADC, as it simply binds to Nectin-4. In the Duravelo study (n=38), which explored patients with metastatic urothelial carcinoma (mUC) treated with chemotherapy/immune checkpoint inhibitors (ICI), the overall response rate (ORR) was 45%, and the median duration of response was 11.1 months. Notably, this drug was associated with lower rates of peripheral neuropathy (36%) compared to EV, which has over 50%, highlighting its potential as a safer option. The Duravelo-2 trial is ongoing and is randomizing patients with treatment-naïve mUC to Zelenectide pevedotin monotherapy or in combination with pembrolizumab. Lastly, other Nectin-4 ADCs with non-MMAE payloads are currently in early-phase studies
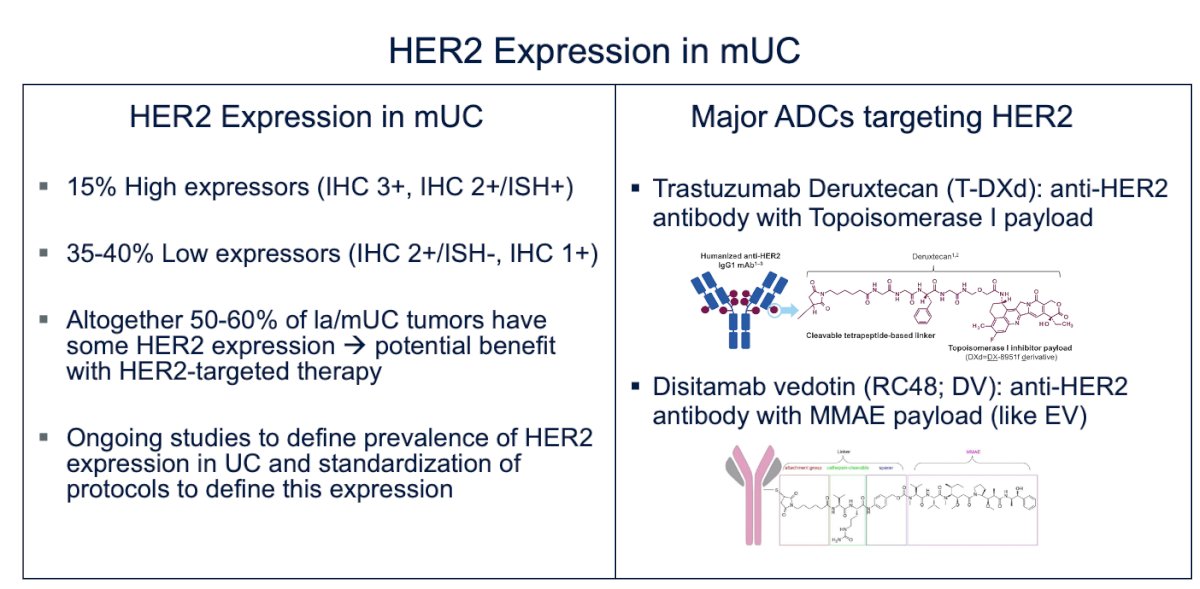
HER2 and its ADC are the other approved targeted therapies for this disease. Evidence shows that 15% of patients are high expressors (IHC 3+, IHC 2+/ISH+), while 35-40% are low expressors (IHC 2+, IHC 1+/ISH-). Altogether, 50-60% of locally advanced or metastatic urothelial carcinoma (mUC) tumors exhibit some level of HER2 expression, suggesting potential benefit from HER2-targeted therapy.
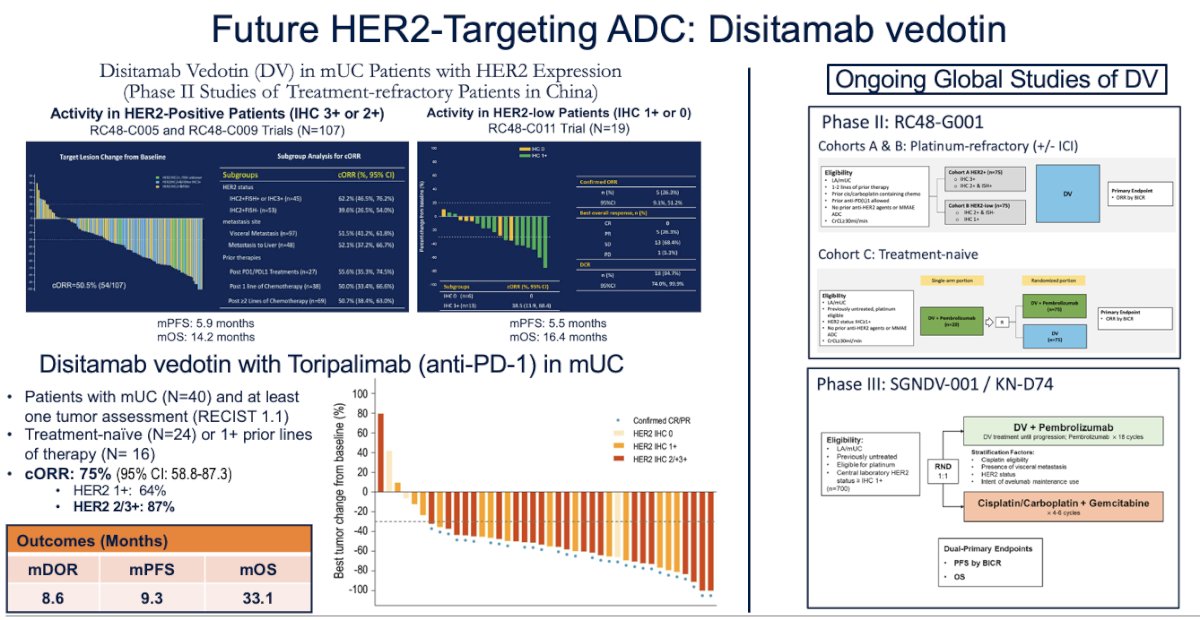
The major ADCs targeting HER2 are trastuzumab deruxtecan, an anti-HER2 antibody with a topoisomerase I payload, and disitamab vedotin, an anti-HER2 antibody with an MMAE payload, similar to enfortumab vedotin (EV)
Trastuzumab deruxtecan was studied in the DESTINY pan-tumor study as monotherapy in HER2-high expressing tumors in patients who had received one or more lines of treatment. This led to the approval of trastuzumab deruxtecan for patients with HER2 mutations, as it demonstrated an impressive overall response rate (ORR) of 40%, with responses enriched in high HER2 expression (56%) in bladder cancer, and a median overall survival (OS) of 13.4 months.2
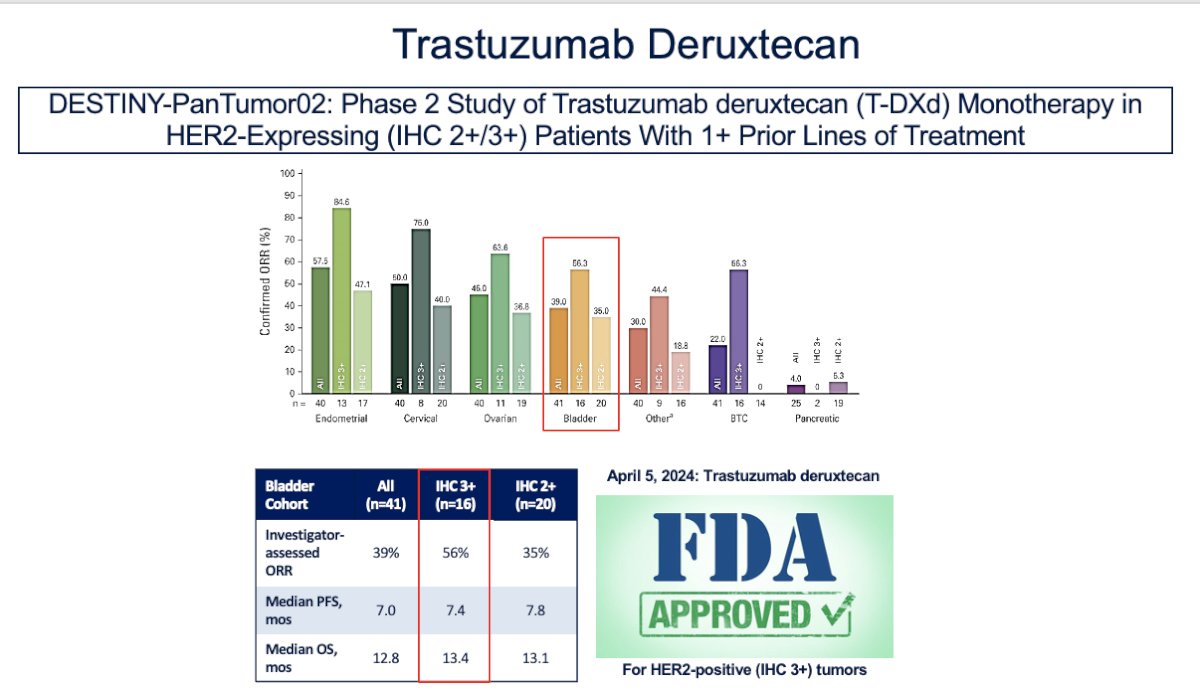
Disitimab Vedotin
This is a drug with advanced research in the metastatic urothelial carcinoma space. However, it originates from China, and most of the data comes from there. The response rates of 50.5% in high HER2 expressors and a median overall survival (OS) of 14.2 months led to the launch of multiple clinical trials exploring this drug in combination with other agents, one of which is toripalimab. The combination of disitamab vedotin (DV) + toripalimab in patients with mUC (24 treatment-naïve and 16 with prior lines of therapy) showed a 75% overall response rate (ORR) and impressive oncological outcomes, with a median OS of 33.1 months, similar to what has been observed with enfortumab vedotin (EV). Dr. Koshkin highlighted that, while this drug has a different target than EV, they share the same MMAE payload. Currently, a Phase III trial is enrolling patients to compare DV + pembrolizumab versus gemcitabine-cisplatin.
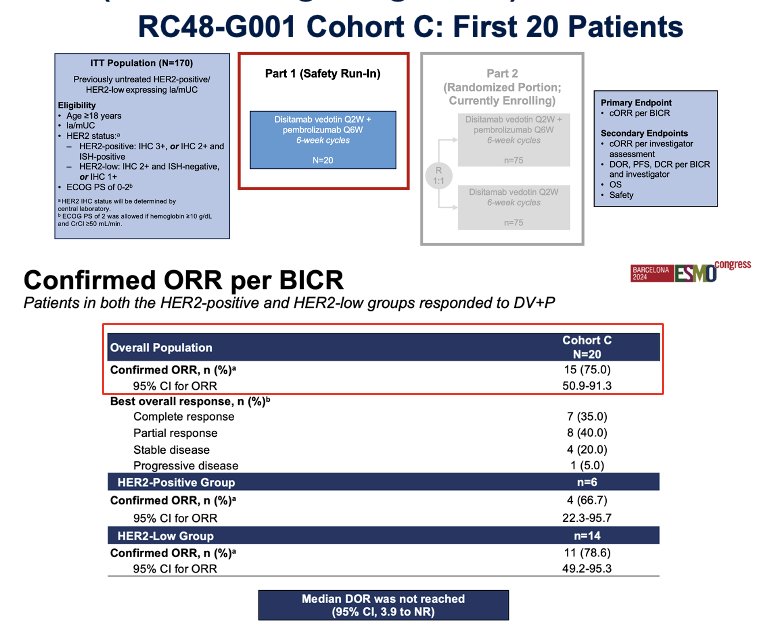
Preliminary data from the Phase III RC48-G001 study, which enrolled 20 patients, reported an overall response rate (ORR) of 75%. While this is impressive data, it is still very early, and we need to wait for the study to complete enrollment before drawing definitive conclusions
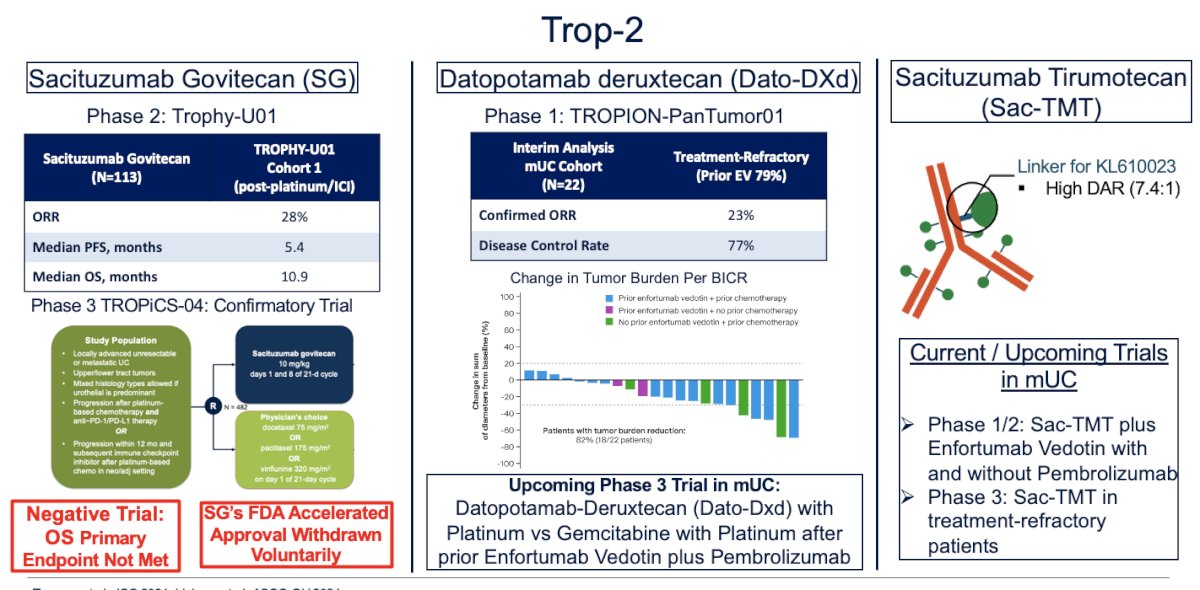
Trop-2
Another target is Trop-2. Sacituzumab govitecan (SG) was initially assessed in the TROPHY-U01 trial, which included a post-platinum/ICI cohort of 113 patients. The overall response rate (ORR) of 28% led to its accelerated approval in 2021. However, the Phase 3 confirmatory TROPiCS-04 trial showed no significant benefit in overall survival (OS) for patients treated with SG, and the primary endpoint was not met, leading to the voluntary withdrawal of SG's FDA accelerated approval.
Other molecules targeting Trop-2, such as datopotamab deruxtecan and sacituzumab tirapazamine, are currently under investigation, with results still awaited
Dual targeting antibodies
Dual-targeting ADCs directed against HER3/EGFR, with a topoisomerase inhibitor payload, have shown promise, achieving an overall response rate (ORR) of 40%, though this was based on a small cohort of 27 patients. Nevertheless, based on these findings, dual ADCs will proceed to larger clinical trials.
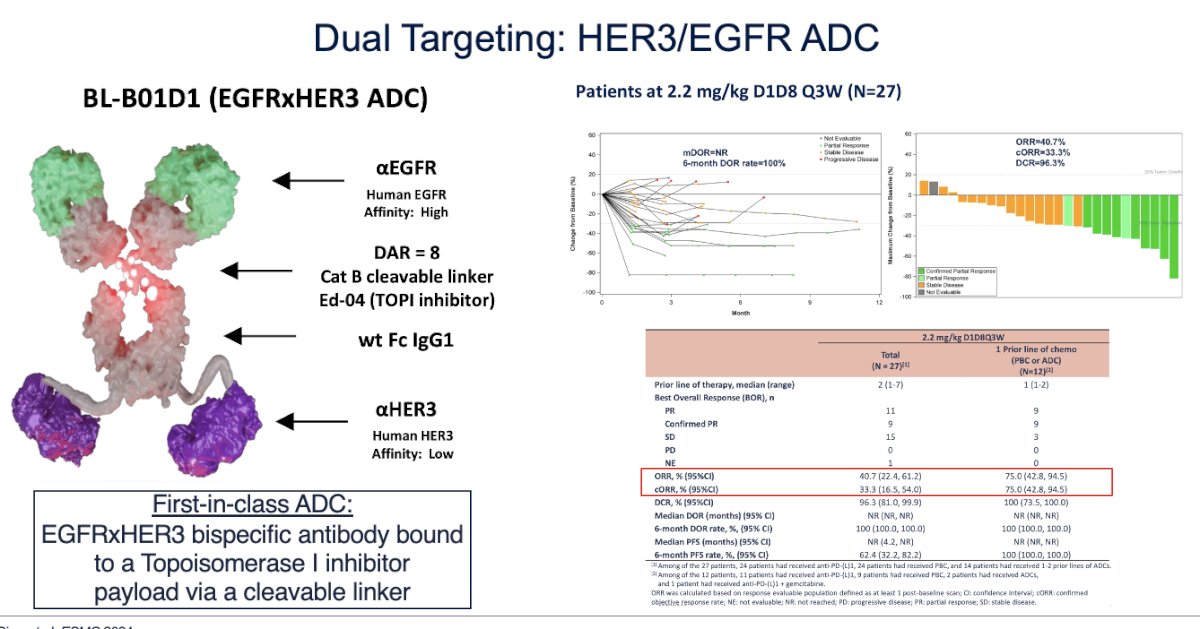
Combination of ADCs with ADCs or ICI
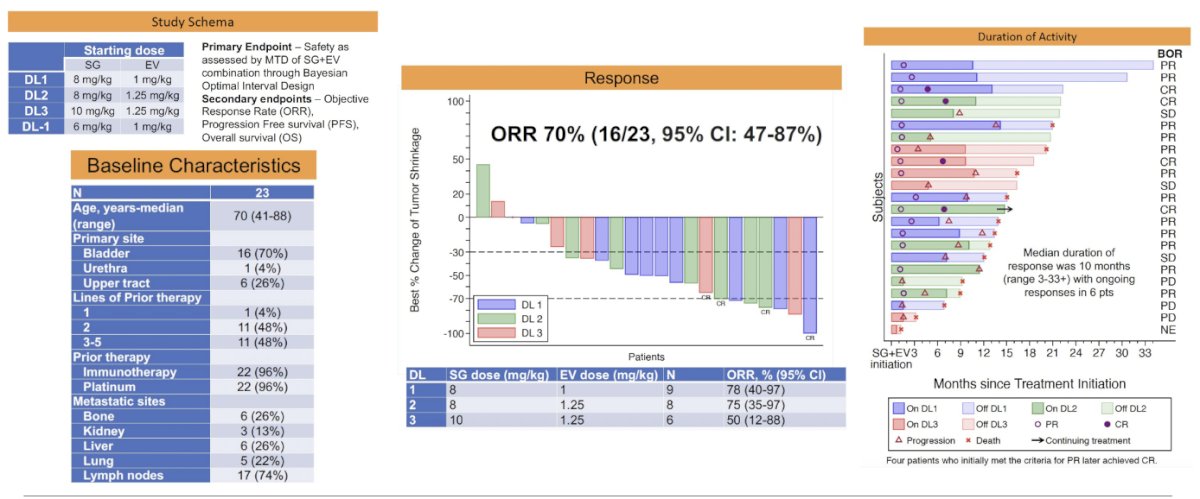
The possibility of combining ADCs has generated significant interest. In a Phase I study of EV + SG in a cohort of 23 patients, the overall response rate (ORR) was 70%, with a median duration of response of 10 months, as shown below.
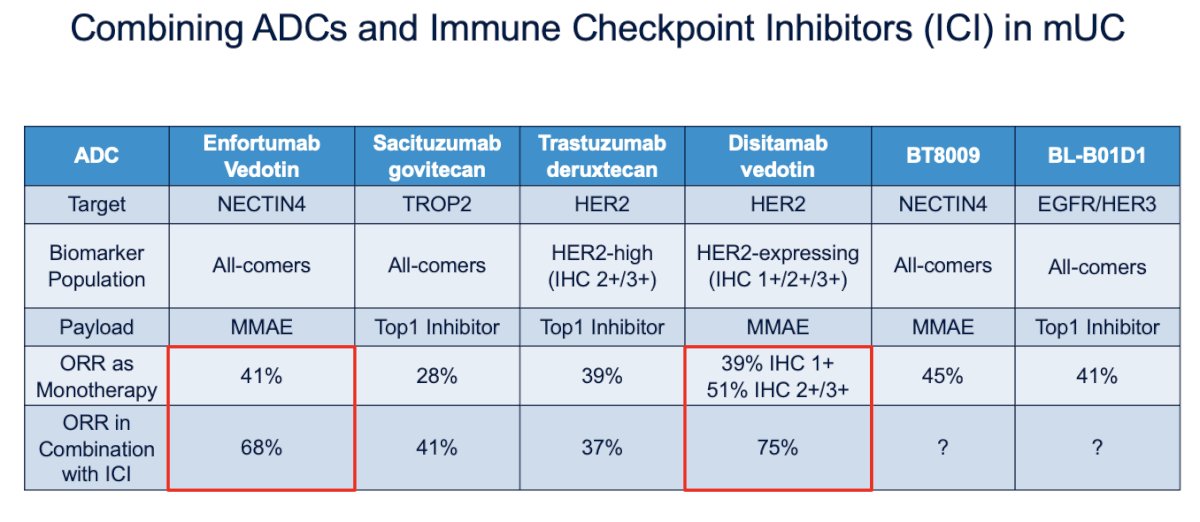
There has also been growing interest in combining ADCs with ICIs in mUC. Below is a summary of the targets, payloads, and ORR for monotherapy and in combination with ICIs, which opens new possibilities for treatment intensification in mUC.
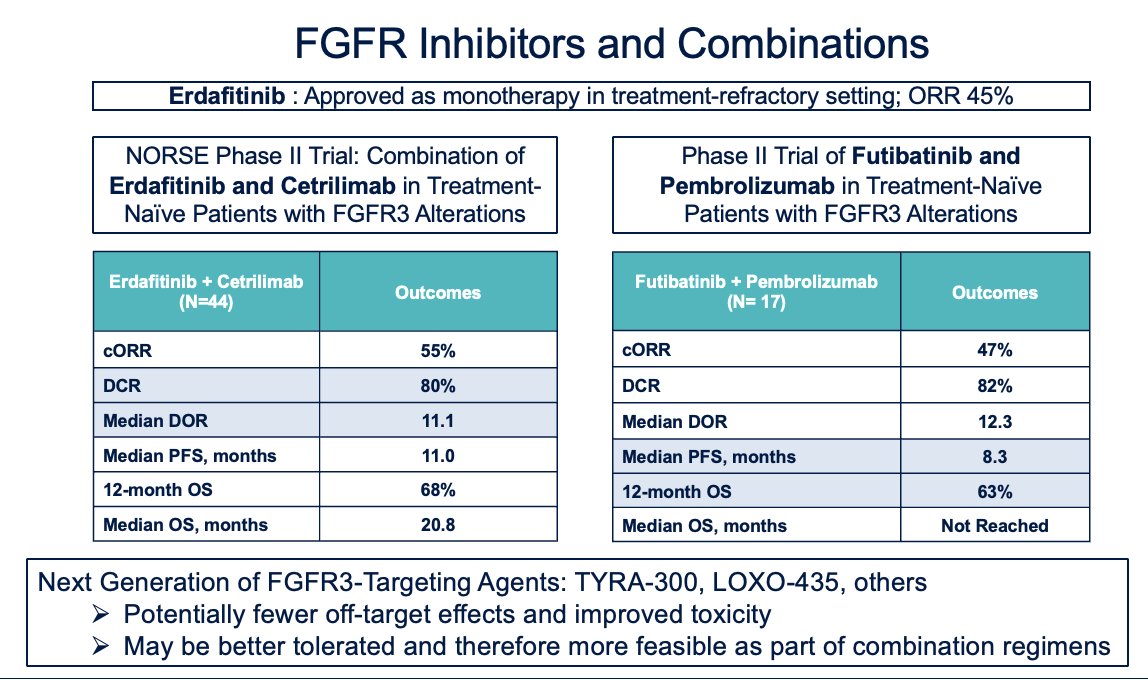
Erdafitinib, the only FGFR inhibitor approved in the refractory setting, has shown an overall response rate (ORR) of around 45% across most trials. Ongoing Phase II trials include the NORSE trial, which is exploring the combination of erdafitinib and cetrilimab in treatment-naïve patients with FGFR3 alterations, and another Phase II trial investigating the combination of futibatinib, a next-generation FGFR3 inhibitor, with pembrolizumab. These next-generation FGFR3 inhibitors potentially have fewer off-target effects and improved toxicity profiles, which may make them better tolerated and more feasible for use in combination regimens
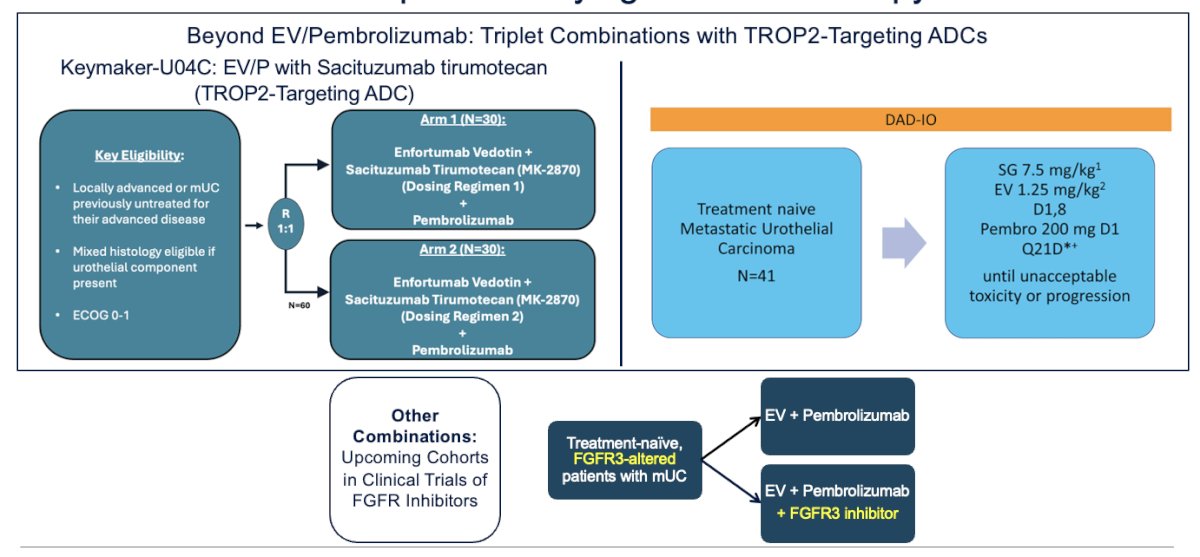
The next steps in the field of mUC are moving beyond the EV + pembrolizumab backbone, building on this foundation with triplet combinations, including TROP-targeting ADCs. One such trial is the DAD-IO trial, which is exploring SG + EV + pembrolizumab. Similarly, some upcoming cohorts in FGFR inhibitor trials will explore intensification with ADCs
Dr. Koshkin wrapped up his presentation with the following messages:
- Significant advances have been made in the urothelial cancer treatment landscape over the past 5-7 years.
- We now have a better understanding of which drugs are effective for this disease, and many new drugs are in development targeting existing mechanisms.
- Targets include Nectin-4, Trop-2, HER2, FGFR3 alterations, and others (e.g., CD86).
- Novel treatment targets are also being explored.
- Future directions will focus on new drug combinations, building on the EV + pembrolizumab backbone.
- Intensifying systemic therapy early in the treatment course for metastatic urothelial carcinoma (mUC), or even earlier in the perioperative setting, will be key
Presented by: Vadim S. Koshkin, MD, Assistant Professor, Division of Hematology and Oncology, Department of Medicine, University of California San Francisco, San Francisco, CA
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 Society of Urologic Oncology (SUO) Annual Meeting, Dallas, TX, Tues, Dec 3 – Fri, Dec 6, 2024.
References:- Powles T, Valderrama BP, Gupta S, Bedke J, Kikuchi E, Hoffman-Censits J, Iyer G, Vulsteke C, Park SH, Shin SJ, Castellano D, Fornarini G, Li JR, Gümüş M, Mar N, Loriot Y, Fléchon A, Duran I, Drakaki A, Narayanan S, Yu X, Gorla S, Homet Moreno B, van der Heijden MS; EV-302 Trial Investigators. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N Engl J Med. 2024 Mar 7;390(10):875-888. doi: 10.1056/NEJMoa2312117. PMID: 38446675.
- Meric-Bernstam F, Makker V, Oaknin A, Oh DY, Banerjee S, González-Martín A, Jung KH, Ługowska I, Manso L, Manzano A, Melichar B, Siena S, Stroyakovskiy D, Fielding A, Ma Y, Puvvada S, Shire N, Lee JY. Efficacy and Safety of Trastuzumab Deruxtecan in Patients With HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial. J Clin Oncol. 2024 Jan 1;42(1):47-58. doi: 10.1200/JCO.23.02005. Epub 2023 Oct 23. PMID: 37870536; PMCID: PMC10730032.


