(UroToday.com) The 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas was host to the Bladder Cancer Course and the Keynote Lecture by Dr. Matthew Galsky who discussed ctDNA-based clinical decision-making in urothelial cancer.
Dr. Galsky began his presentation by emphasizing that perioperative systemic therapy is one of the greatest achievements in solid tumor medical oncology. However, he cautioned that in the future, our current approach may be viewed critically, as we are not selecting patients appropriately. This lack of proper selection, he argued, contributes to the phenomenon of “imprecision medicine.
In the EORTC 30994 trial, an open label, randomised, phase 3 trial evaluating adjuvant chemotherapy (four cycles of gemcitabine plus cisplatin, high-dose methotrexate, vinblastine, doxorubicin, and cisplatin [high-dose MVAC], or MVAC) in pT3-pT4 disease or node positive (pN1-3) M0 disease after radical cystectomy. Interestingly and as shown in the graphic below, there is a considerable group of patients who don’t need treatment and a group of patients who need treatment but don’t benefit from it.
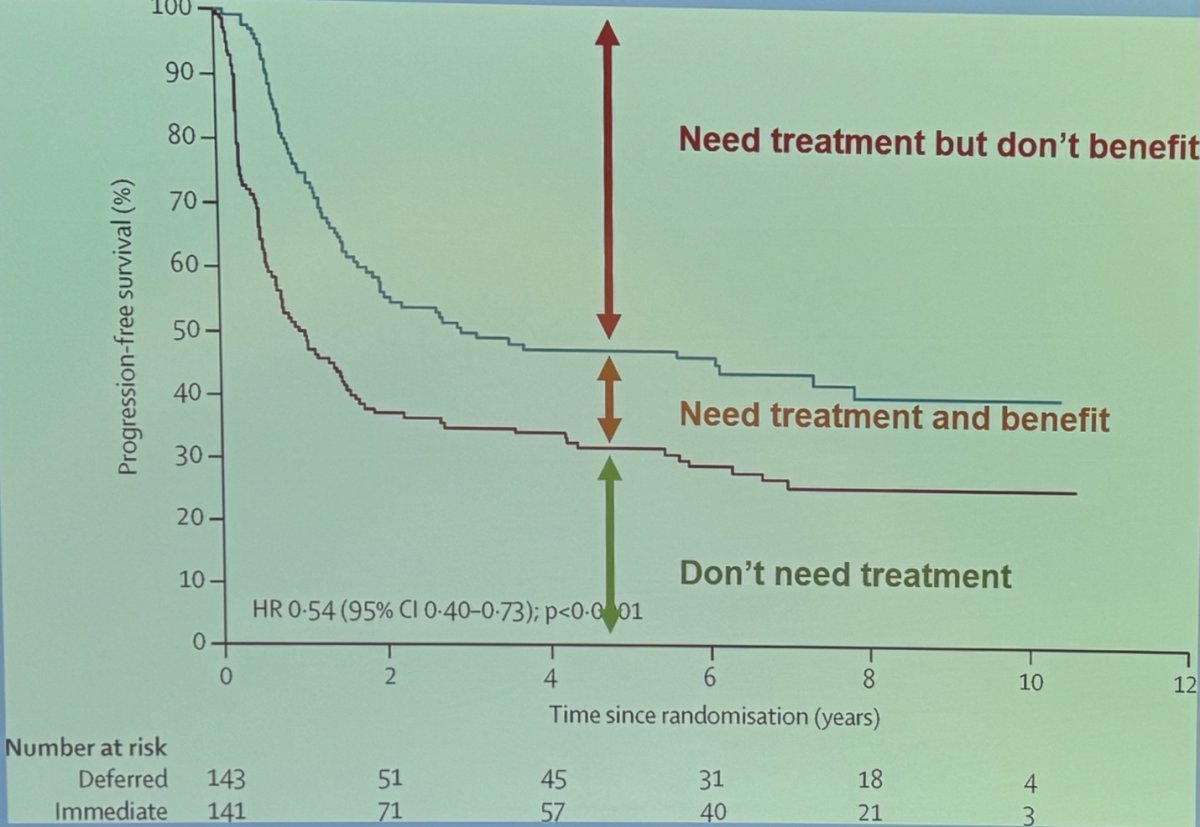
Dr. Galsky discussed the "double biomarker dilemma," which involves identifying patients who need treatment and determining who among these patients will actually benefit from it. He highlighted two main groups of patients:
- Those who would benefit from systemic therapy but do not need it (i.e., they have no micrometastatic disease), and
- Those who need systemic therapy but do not benefit from it.
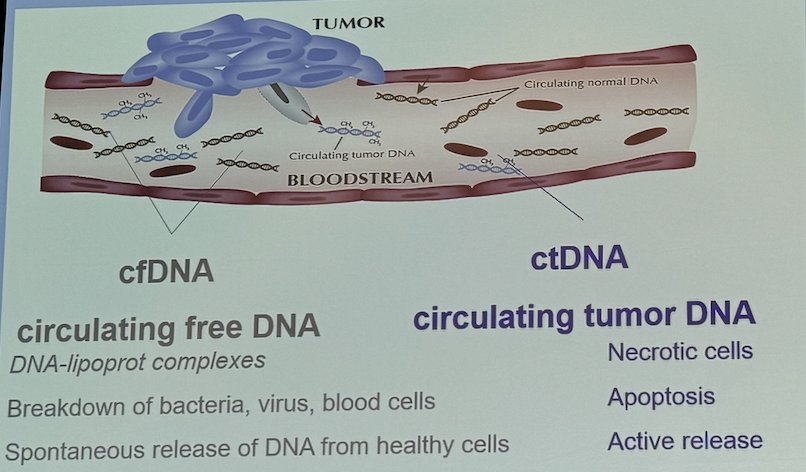
Circulating free DNA (cfDNA) consists of DNA-lipoprotein complexes, breakdown products from bacteria, viruses, and blood cells, as well as spontaneously released DNA from healthy cells. Typically, DNA represents less than 0.1% of total circulating free cfDNA, with a half-life of less than 2 hours. This short half-life offers a window for real-time tumor burden monitoring, but it also complicates its clinical use. On the other hand, circulating tumor DNA (ctDNA) is released from necrotic cells, apoptosis, or even active release. Therefore, ctDNA has the potential to be used as a measure to determine who needs treatment.
The evolution of circulating tumor DNA (ctDNA) for minimal residual disease (MRD) testing spans several decades. It began in 1948 when cfDNA was first identified. In 1977, higher concentrations of cfDNA were found in patients with cancer, suggesting its potential use as a biomarker. Finally, in 2008, Luis Diaz refined the concept of ctDNA, proposing its use to assess tumor dynamics.2
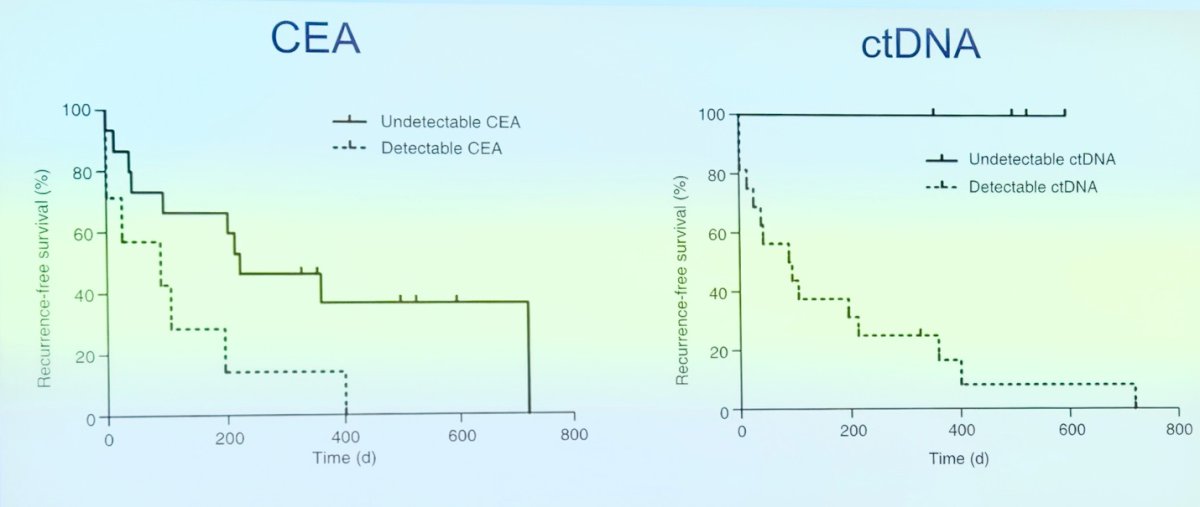
Parallel to the development of carcinoembryonic antigen (CEA) for colon cancer, ctDNA was also being studied as a marker of MRD. The recurrence-free survival curves with ctDNA were impressive (shown below) when compared to the most commonly used biomarker of MRD in colon cancer back in 2008. However, due to the need for different stages of development for biomarkers, ctDNA was not introduced or routinely used in clinical practice at that time.
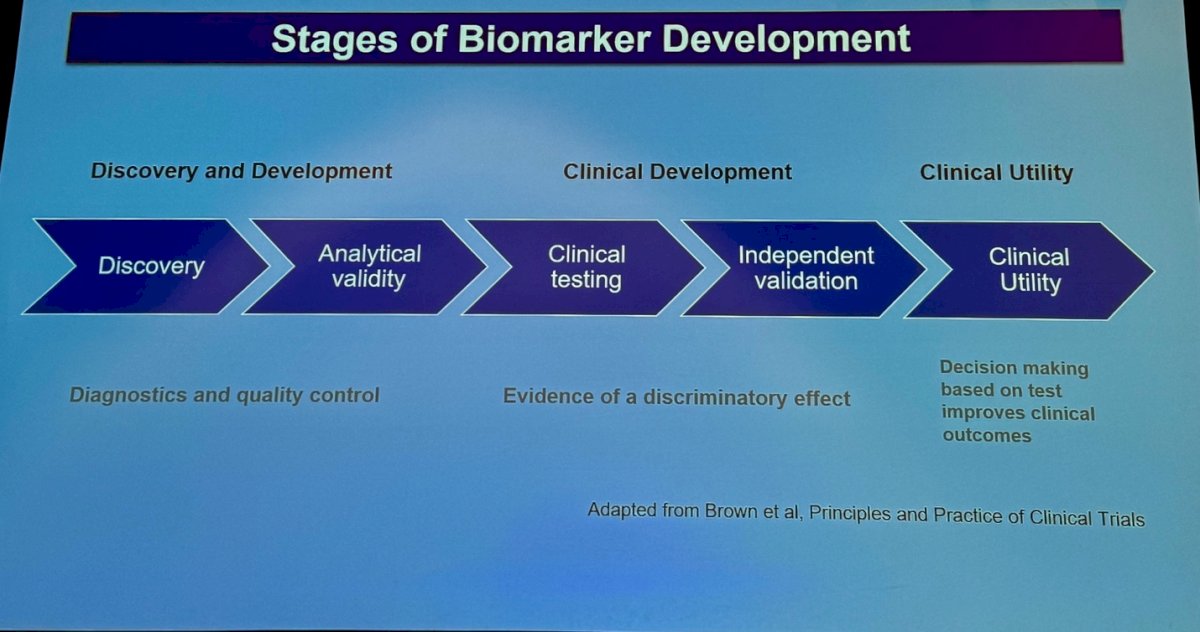
The stages of biomarker development can be divided into three main categories as shown in the figure below. Briefly, the process begins with the discovery of the biomarker, followed by determining its analytical validity and performing quality control. The next phase is clinical development, which includes clinical testing and independent validations. Finally, the biomarker is used in clinical practice as part of the decision-making process to potentially improve clinical outcomes. This progression ensures that the biomarker is not only scientifically sound but also clinically applicable and beneficial for patient care. Currently, with ctDNA we are in the independent validation space and close to using it in the decision-making process and asses its clinical utility.
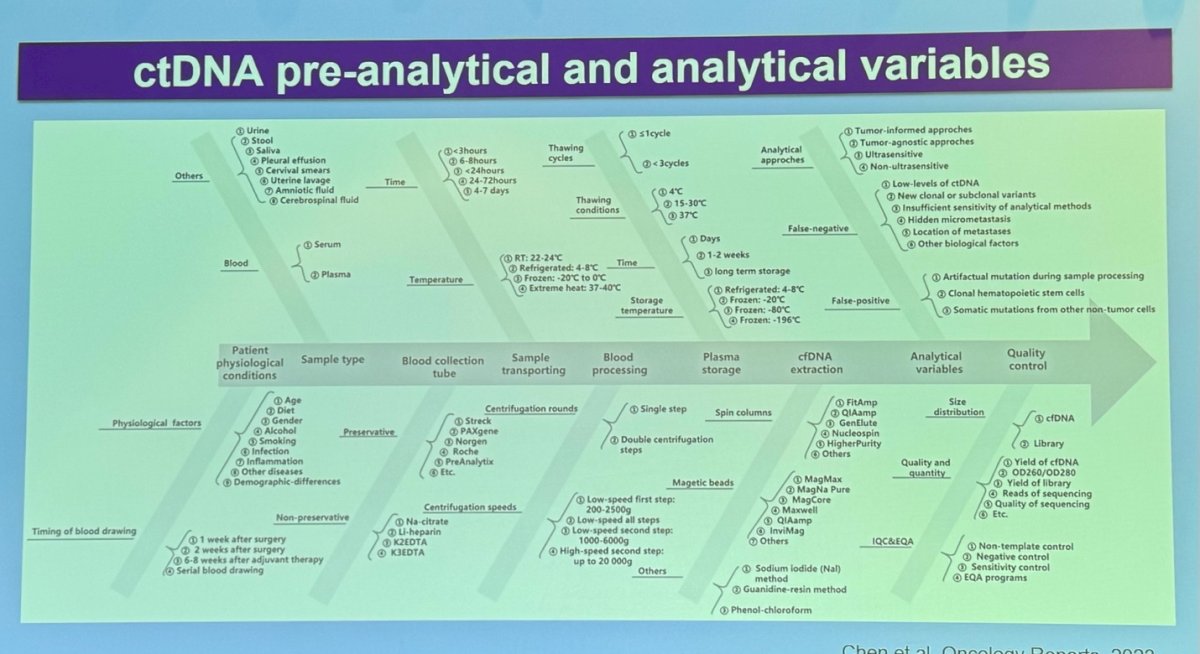
For ctDNA specifically, the preanalytical and analytical variables have many ramifications and require tuning of various factors. These include the source of the ctDNA, physiological factors, the timing of sample collection, the type of collection tubes, the recipients, and storage conditions. After these preanalytical variables are controlled, the process moves on to analytical approaches and performing quality control on the samples. Each step is crucial to ensure the reliability and accuracy of ctDNA as a biomarker in clinical practice.
There are two major approaches to ctDNA-based MRD detection
- Tumor informed: using the tumor to build the ctDNA signature (higher sensitivity)
- Tumor agnostic: It has a faster turn-around time, lower cost, and the ability to detect emerging resistant mutations.
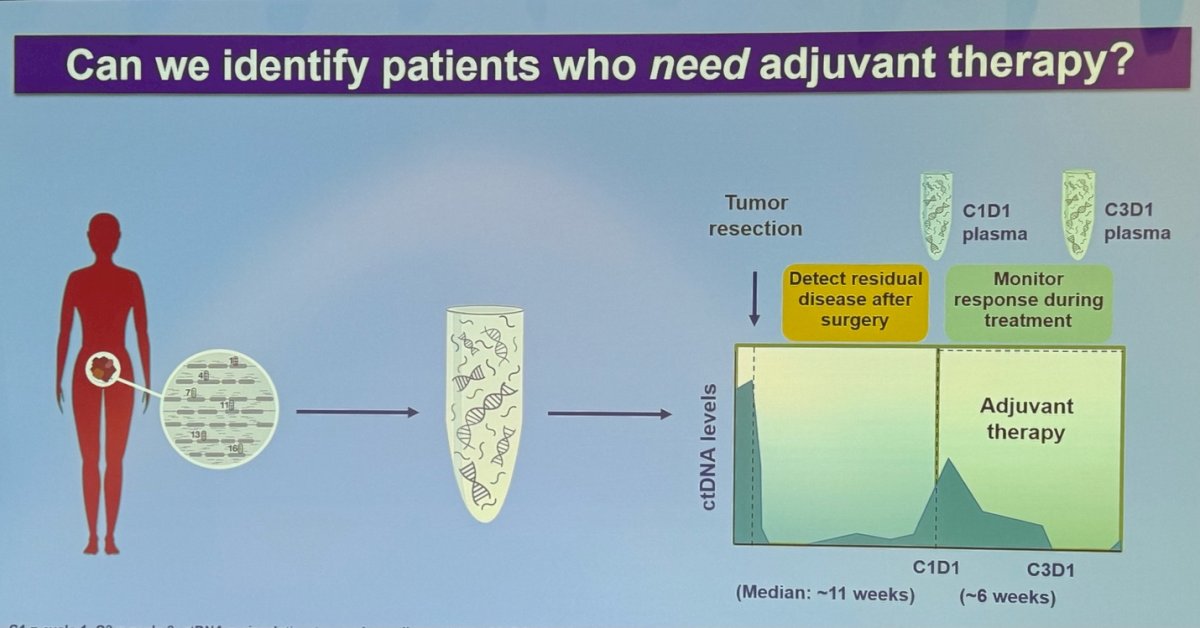
Dr. Galsky discussed the potential of using ctDNA to identify patients who need adjuvant therapy in urothelial carcinoma. He emphasized the importance of establishing baseline ctDNA levels before surgery, then using ctDNA to detect MRD after surgery and to monitor treatment response during adjuvant therapy. This approach allows for more precise identification of patients who may benefit from additional treatment, optimizing patient outcomes.
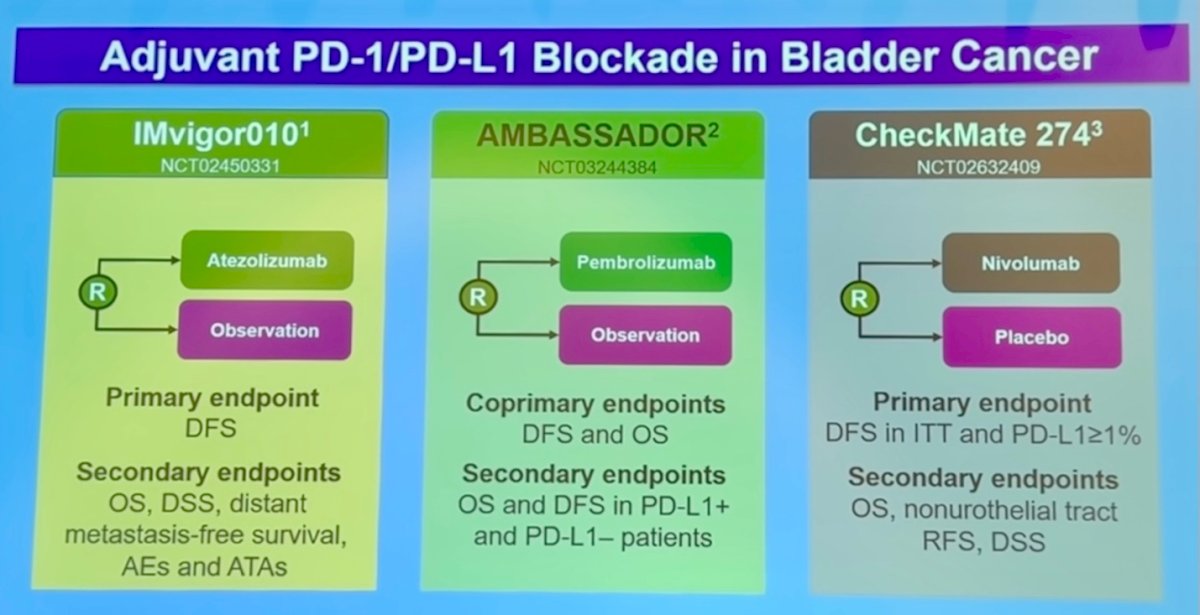
There are three major trials exploring adjuvant PD-1/PD-L1 blockade in bladder cancer: IMvigor010 (Atezolizumab), AMBASSADOR (Pembrolizumab), and CheckMate 274 (Nivolumab). So the question is can we better identift patients who need adjuvant therapy in bladder cancer?
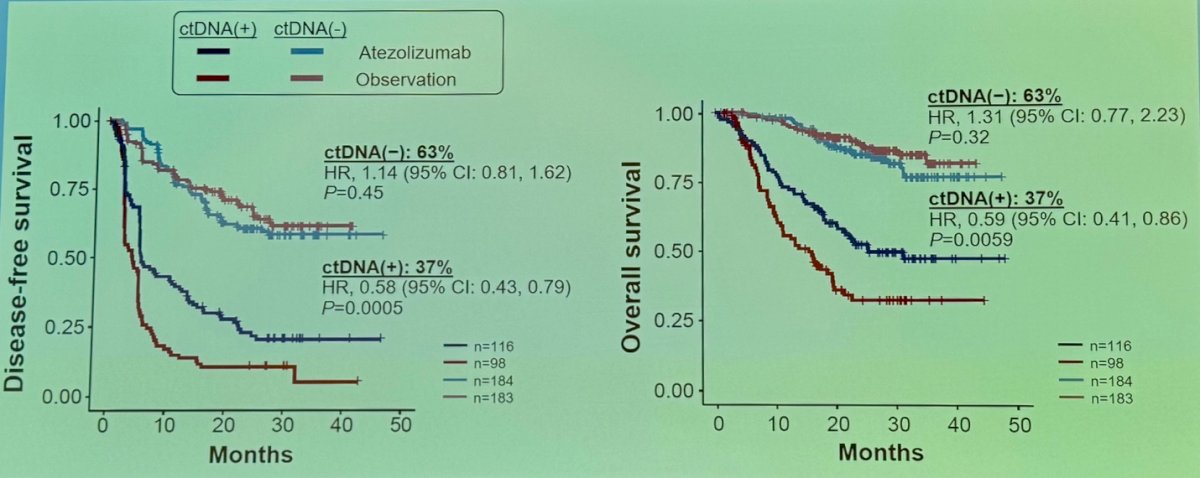
Dr. Galsky presented an exploratory analysis of the IMvigor010 study, a randomized phase III trial comparing adjuvant atezolizumab versus observation. The investigators performed ctDNA testing at the start of therapy. Notably, ctDNA-positive patients had improved disease-free survival (DFS) and overall survival (OS) in the atezolizumab arm compared to the observation arm.3 There was no difference in DFS or OS between treatment arms for ctDNA-negative patients as shown in the Kaplan Meier curves below:
Dr. Galsky mentioned two major phase 3 study designs using ctDNA as a biomarker in the adjuvant setting. One design involves using ctDNA for MRD testing and then randomizing patients based on the test result. The second design uses ctDNA for MRD testing and randomizes patients based on whether they are MRD-positive or MRD-negative, as shown in the graphic below.

One example of the study design randomizing patients based on the test result is the DYNAMIC study, this study focuses on Stage II colon cancer, utilizing ctDNA as a biomarker. In this study, patients are randomized into two arms: one arm receives ctDNA-guided management, while the other arm receives standard management.4 The study design is shown below.
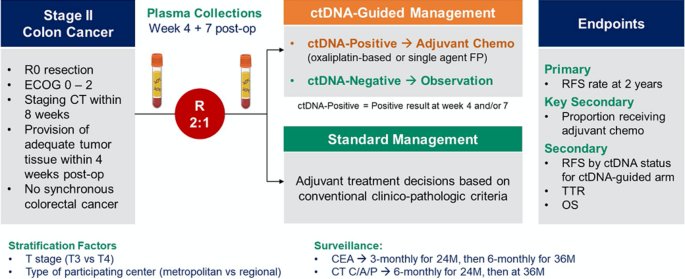
In the DYNAMIC study, at the 5-year follow-up, ctDNA-guided management was not inferior to standard management in terms of disease-free survival (DFS) or overall survival (OS). The 5-year OS for the ctDNA-guided treatment arm was 93.8%, compared to 93.3% for the standard management arm.4
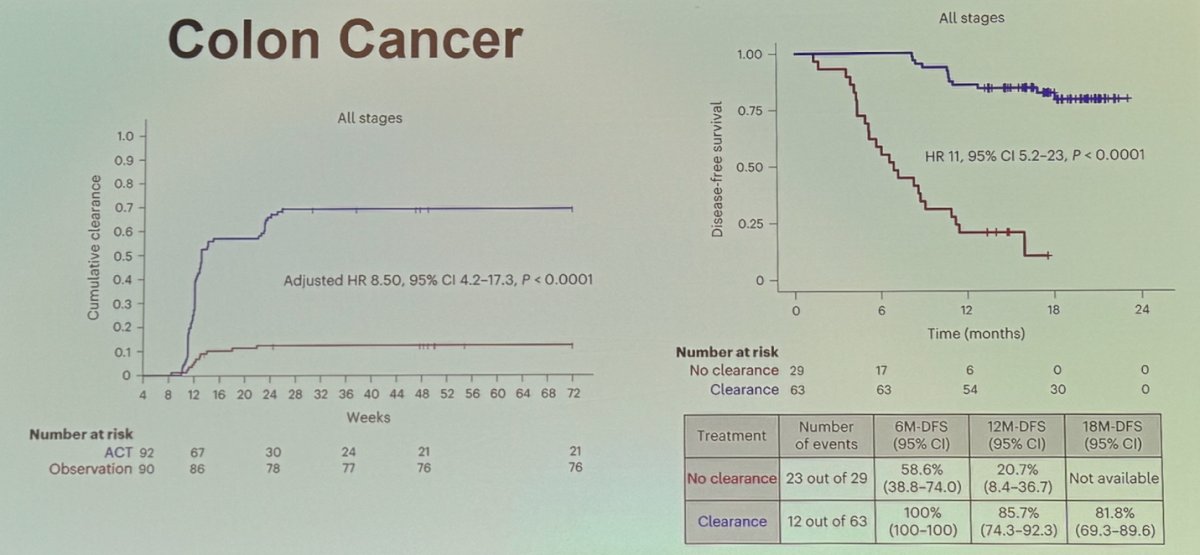
Next steps involve determining whether ctDNA-based MRD could serve as an intermediate clinical endpoint for clinical trials. In colon cancer, an exploratory analysis from GALAXY, an observational arm of the ongoing CIRCULATE-Japan study, analyzed presurgical and postsurgical ctDNA in patients with stage II-IV resectable colorectal cancer. The analysis identified that ctDNA clearance was significantly associated with improved disease-free survival (DFS), as shown below:
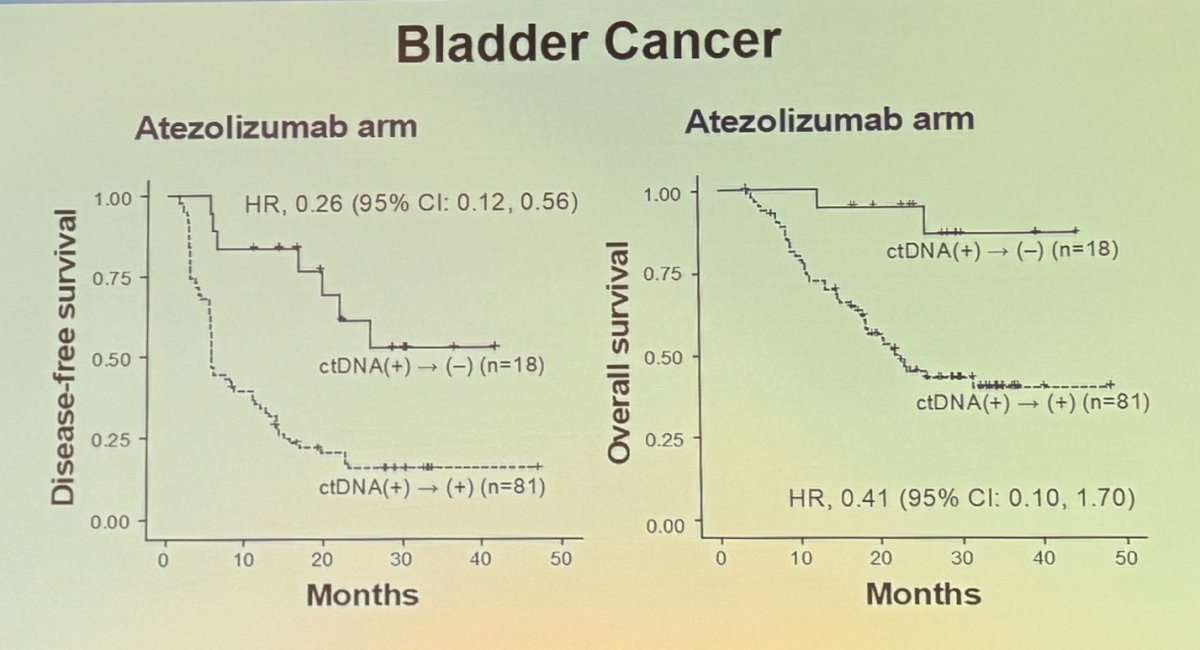
Dr. Galsky continued by discussing the potential of ctDNA-based MRD as an intermediate clinical endpoint in bladder cancer. He presented the analysis from the IMvigor010 trial, which showed that patients who achieved clearance of ctDNA (i.e., transitioned from ctDNA-positive to ctDNA-negative) had significantly better disease-free survival (DFS) (HR 0.26) and overall survival (OS) (HR 0.41).
The FDA recently released draft guidance for the industry on the use of ctDNA in early-stage solid tumor drug development. They recommended that ctDNA could be used as a measure of response. However, while using ctDNA as an early endpoint in clinical trials shows potential, further data is required to support its use as a reliable endpoint for ctDNA clearance.
Dr. Galsky discussed the TOMBOLA study, a national, non-randomized ctDNA-based intervention study conducted at five centers in Denmark. The study included eligible patients with cT2-4aN0-1M0, cisplatin- and immunotherapy-eligible MIBC who underwent neoadjuvant chemotherapy (NAC) followed by radical cystectomy. Patients underwent serial ctDNA testing post-operatively, and upon detection of ctDNA, they were recommended for one year of atezolizumab therapy along with continued serial ctDNA testing.
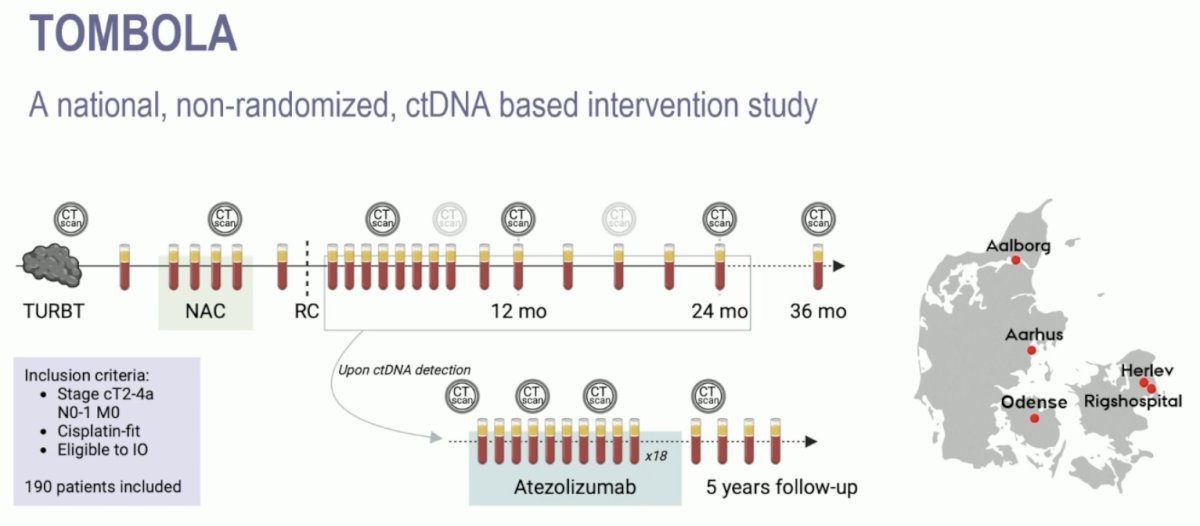
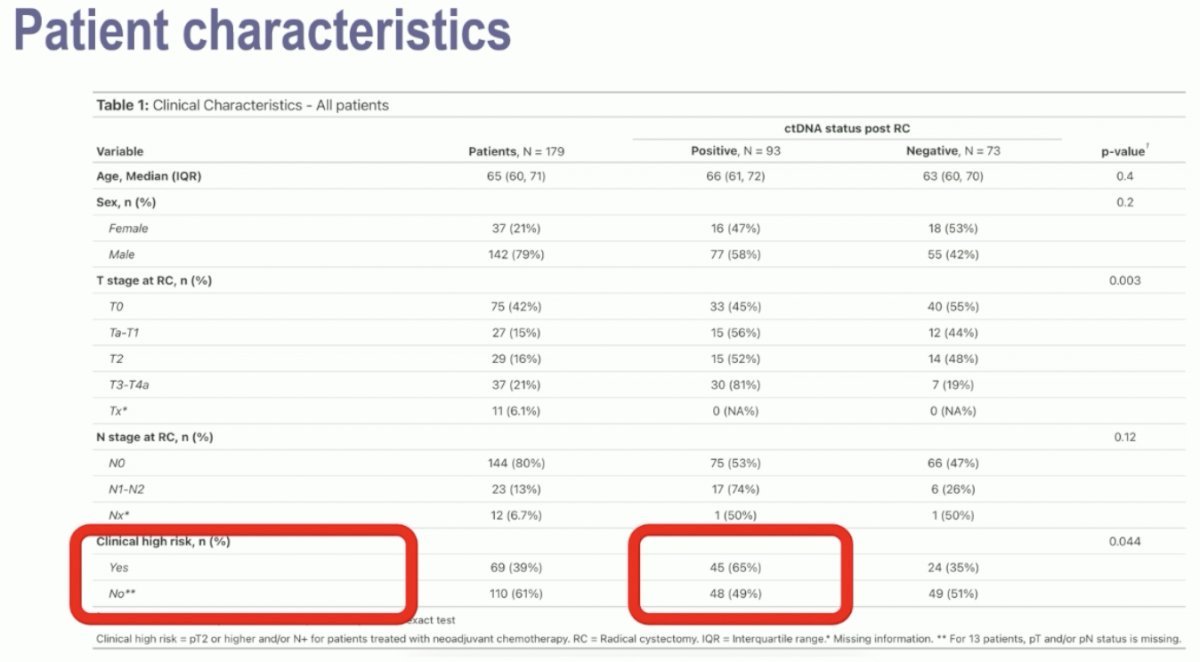
Notably, 39% and 61% of patients had clinical high- and low-risk disease. Notably, among the clinical high-risk patients, 65% had a positive ctDNA status post-radical cystectomy. Conversely, almost half (49%) of the ‘low-risk’ patients were also ctDNA+ post-operatively.
A total of 93 patients (56%) were ctDNA+ post-Radical cystectomy, of these 75% were detected less than 4 months post-surgery. Notably, of the cDNA- patients, only 2 (3%) developed metastases on CT-scan during follow-up. Dr. Galsky mentioned that 55% of the 44 patients with a ctDNA+ status converted to ctDNA- with no evidence of disease (NED) on imaging.
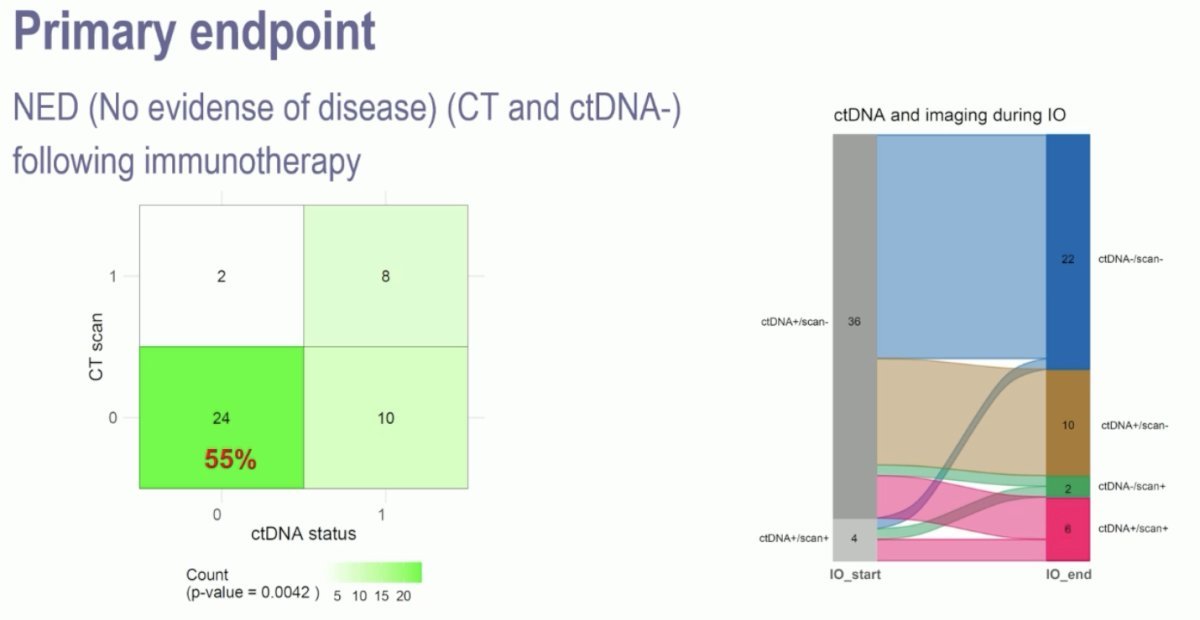
One of the notable findings from the TOMBOLA study was that it included patients with ≥ cT2 disease, 42% of the overall population, and 55% of the ctDNA-negative group were pT0 after cystectomy. The study also found a higher risk of ctDNA positivity in patients with higher-risk features. Additionally, there was a relatively high rate of ctDNA clearance compared to IMvigor010. However, since the study did not include randomization, it raises the question: Is surveillance as effective as adjuvant immunotherapy in patients with poor adverse pathological features who have a ctDNA-negative result after cystectomy?
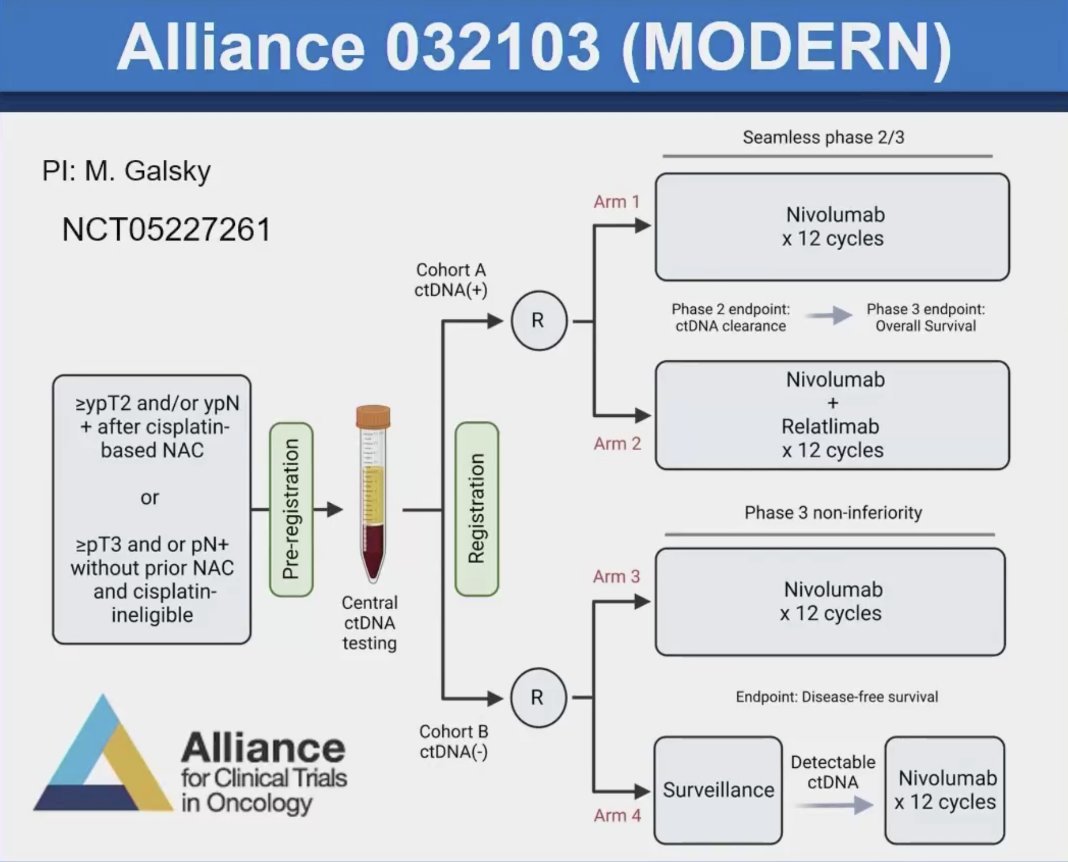
Dr. Galsky highlighted the trial schema of the Alliance 032103 MODERN study, which involves bladder cancer patients who have undergone cystectomy. In this study, ctDNA status is used for randomization, exemplifying the "randomize to the result" design. Patients in the ctDNA-positive group will receive either nivolumab or nivolumab plus relatlimab. Those in the ctDNA-negative group will receive either standard of care (adjuvant nivolumab for 12 cycles) or surveillance. If ctDNA becomes detectable during surveillance, they will receive adjuvant nivolumab.
The known unknowns of ctDNA to date are:
- What are the implications of cDNA+ in the pre-surgical setting?
- Is MRD a qualitative versus quantitative biomarker?
- When is the right time to test post-surgery?
- Transient versus sustained cDNA clearance?
- Is molecular recurrence a new clinical disease state and can studies be feasibly conducted?
Dr Galsky delved briefly trying to answer some of these questions. He mentioned that quantitative measures of ctDNA have prognostic implications and that pre-surgical ctDNA levels versus post-surgical ctDNA clearance can be quantified and help to inform MRD clearance. Similarly in the adjuvant chemotherapy/IO setting, ctDNA can help to inform clearance of ctDNA while on treatment.
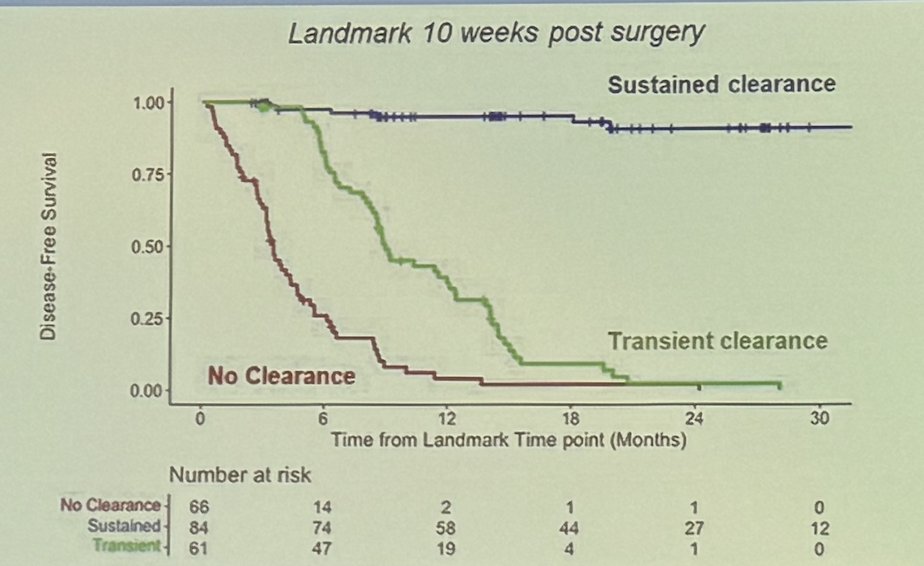
Dr. Galsky emphasized that not all ctDNA clearance is the same, citing the landmark analysis of ctDNA dynamics from MRD detection in the GALAXY study (Colorectal cancer). The analysis revealed that patients with sustained ctDNA clearance had significantly better outcomes compared to those with transient ctDNA clearance (HR: 32.57, 95% CI: 9.94–106.76, p < 0.0001) or those with no clearance at all.
Dr. Galsky concluded his presentation with the following key takeaways:
- ctDNA as a measure of MRD is poised to change the perioperative treatment paradigm in bladder cancer
- Prospective studies are required to establish clinical utility before employing ctDNA for routine clinical decision-making
Presented by: Matthew Galsky, MD, Director of Genitourinary Medical Oncology at Tisch Cancer Institute/Mount Sinai School of Medicine, New York, United States of America.
Written by: Julian Chavarriaga, MD –Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 Society of Urologic Oncology (SUO) Annual Meeting, Dallas, TX, Tues, Dec 3 – Fri, Dec 6, 2024.
References:- Sternberg CN, Skoneczna I, Kerst JM, Albers P, Fossa SD, Agerbaek M, Dumez H, de Santis M, Théodore C, Leahy MG, Chester JD, Verbaeys A, Daugaard G, Wood L, Witjes JA, de Wit R, Geoffrois L, Sengelov L, Thalmann G, Charpentier D, Rolland F, Mignot L, Sundar S, Symonds P, Graham J, Joly F, Marreaud S, Collette L, Sylvester R; European Organisation for Research and Treatment of Cancer Genito-Urinary Cancers Group; Groupe d'Etude des Tumeurs Urogénitales; National Cancer Research Institute Bladder Cancer Study Group; National Cancer Institute of Canada Clinical Trials Group; German Association of Urologic Oncology. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. Lancet Oncol. 2015 Jan;16(1):76-86. doi: 10.1016/S1470-2045(14)71160-X. Epub 2014 Dec 11. PMID: 25498218.
- Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, Thornton K, Agrawal N, Sokoll L, Szabo SA, Kinzler KW, Vogelstein B, Diaz LA Jr. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008 Sep;14(9):985-90. doi: 10.1038/nm.1789. Epub 2007 Jul 31. PMID: 18670422; PMCID: PMC2820391.
- Powles T, Assaf ZJ, Davarpanah N, Banchereau R, Szabados BE, Yuen KC, Grivas P, Hussain M, Oudard S, Gschwend JE, Albers P, Castellano D, Nishiyama H, Daneshmand S, Sharma S, Zimmermann BG, Sethi H, Aleshin A, Perdicchio M, Zhang J, Shames DS, Degaonkar V, Shen X, Carter C, Bais C, Bellmunt J, Mariathasan S. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature. 2021 Jul;595(7867):432-437. doi: 10.1038/s41586-021-03642-9. Epub 2021 Jun 16. PMID: 34135506.
- Tie J, Cohen JD, Lahouel K, Lo SN, Wang Y, Kosmider S, Wong R, Shapiro J, Lee M, Harris S, Khattak A, Burge M, Harris M, Lynam J, Nott L, Day F, Hayes T, McLachlan SA, Lee B, Ptak J, Silliman N, Dobbyn L, Popoli M, Hruban R, Lennon AM, Papadopoulos N, Kinzler KW, Vogelstein B, Tomasetti C, Gibbs P; DYNAMIC Investigators. Circulating Tumor DNA Analysis Guiding Adjuvant Therapy in Stage II Colon Cancer. N Engl J Med. 2022 Jun 16;386(24):2261-2272. doi: 10.1056/NEJMoa2200075. Epub 2022 Jun 4. PMID: 35657320; PMCID: PMC9701133.
- Kotani D, Oki E, Nakamura Y, Yukami H, Mishima S, Bando H, Shirasu H, Yamazaki K, Watanabe J, Kotaka M, Hirata K, Akazawa N, Kataoka K, Sharma S, Aushev VN, Aleshin A, Misumi T, Taniguchi H, Takemasa I, Kato T, Mori M, Yoshino T. Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer. Nat Med. 2023 Jan;29(1):127-134. doi: 10.1038/s41591-022-02115-4. Epub 2023 Jan 16. PMID: 36646802; PMCID: PMC9873552.


