(UroToday.com) The 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, between December 3 and December 6, 2024, was host to the Bladder Cancer Course and the session Metastatic Urothelial Cancer. Dr. Tracy Rose discussed the Evolution of First-Line Treatment for Metastatic Urothelial Cancer.
Dr. Rose began her presentation by sharing that the treatment landscape in the first-line of metastatic urothelial carcinoma (mUC) has changed significantly in recent years.
In 2000, gemcitabine plus cisplatin (GC) and methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) were compared in patients with mUC in a phase III multicenter study. GC had comparable efficacy and improved toxicity compared to MVAC. The overall response rate was 49.4% for GC versus 45.7% for MVAC, with a median overall survival (OS) of 13.8 months for GC versus 14.8 months for MVAC. However, GC had a lower drug mortality rate (1%) compared to MVAC (3%). GC also showed higher rates of anemia and thrombocytopenia, while MVAC had higher rates of febrile neutropenia and mucositis.1
One year after a phase III trial comparing high-dose-intensity chemotherapy with methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) plus granulocyte colony-stimulating factor (HD-MVAC) versus MVAC in patients with mUC, increasing the dose intensity of cisplatin to q2w with growth factor support resulted in a significant improvement in the ORR. The ORR was 62% for HD-MVAC compared to 50% for MVAC, with a similar toxicity profile (treatment-related deaths were 3% for HD-MVAC and 4% for MVAC). Since then, ddMVAC has become the standard of care GC alternative.2
The clinical dilemma we still face is that many patients with mUC are cisplatin-ineligible. This ineligibility is due to various factors, including ECOG-PS ≥2, creatinine clearance <60mL/min, hearing loss≥2, neuropathy ≥2, and NYHA class III heart failure.
In 2006, an option for cisplatin-ineligible patients was introduced with the EORTC 30986 study. This study randomized patients ineligible for cisplatin (GFR between 30-60 mL/min or ECOG-PS ≥2) to receive either gemcitabine/carboplatin or methotrexate/carboplatin/vinblastine (M-CAVI). The overall response rate was 41.2% for the Gem/Carb arm compared to 30.3% for the M-CAVI arm (P = .08). The median OS was 9.3 months in the GC arm and 8.1 months in the M-CAVI arm.3
Platinum-based chemotherapy has remained the standard of care in the first-line setting for mUC for decades, with overall response rates (ORR) ranging from 31% with Gem/Carbo to 63% with ddMVAC.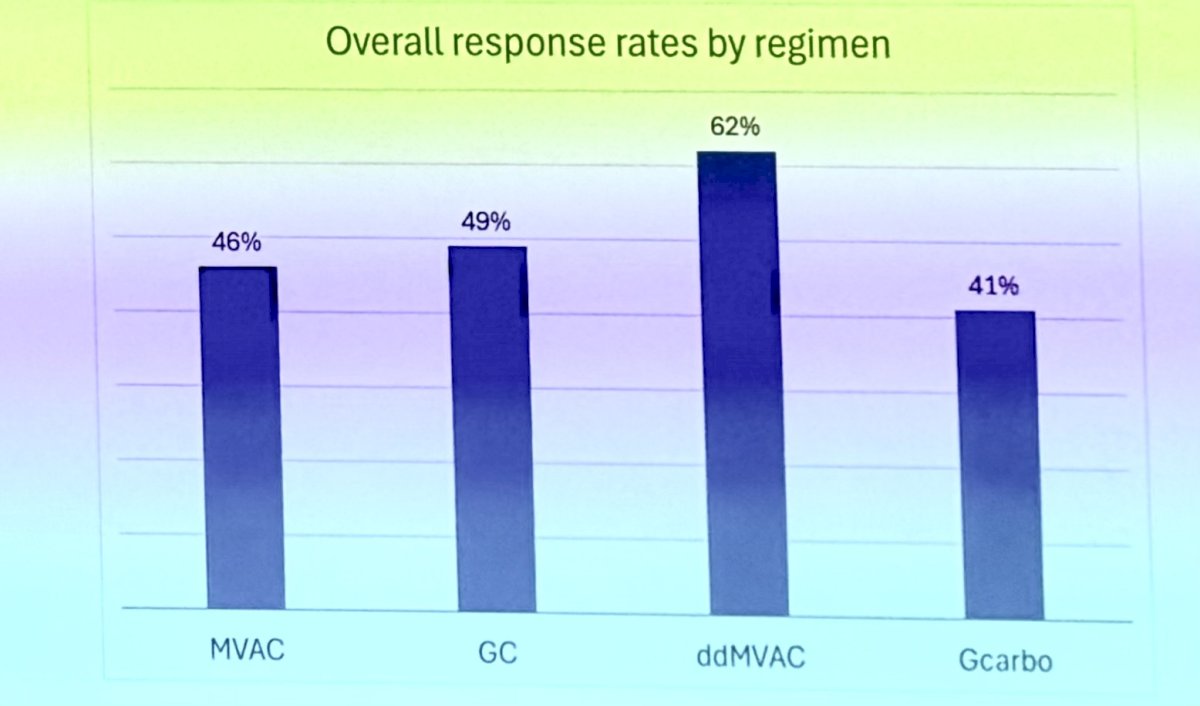
However, despite the ORR in platinum-based combination chemotherapy regimens, the median overall survival (OS) remains less than 18 months regardless of the regimen. The median OS is 9.3 months with Gem/Carbo and 15.5 months with ddMVAC.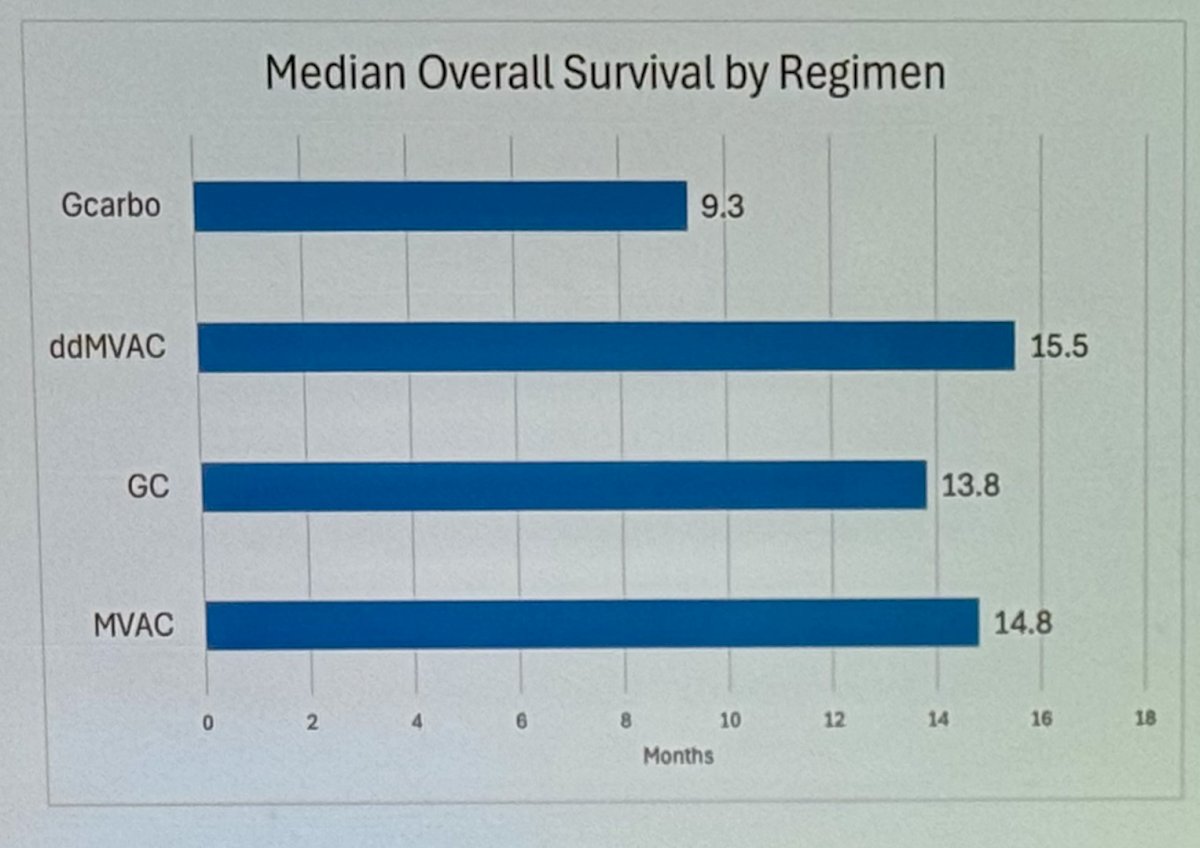
The mUC treatment paradigm until 2017 was relatively simple, primarily considering whether patients were cisplatin-eligible or cisplatin-ineligible. Treatment options included various chemotherapy combinations, as shown below. Patients with a complete or partial response, or stable disease, would proceed to surveillance, while those with progression would receive second-line therapy.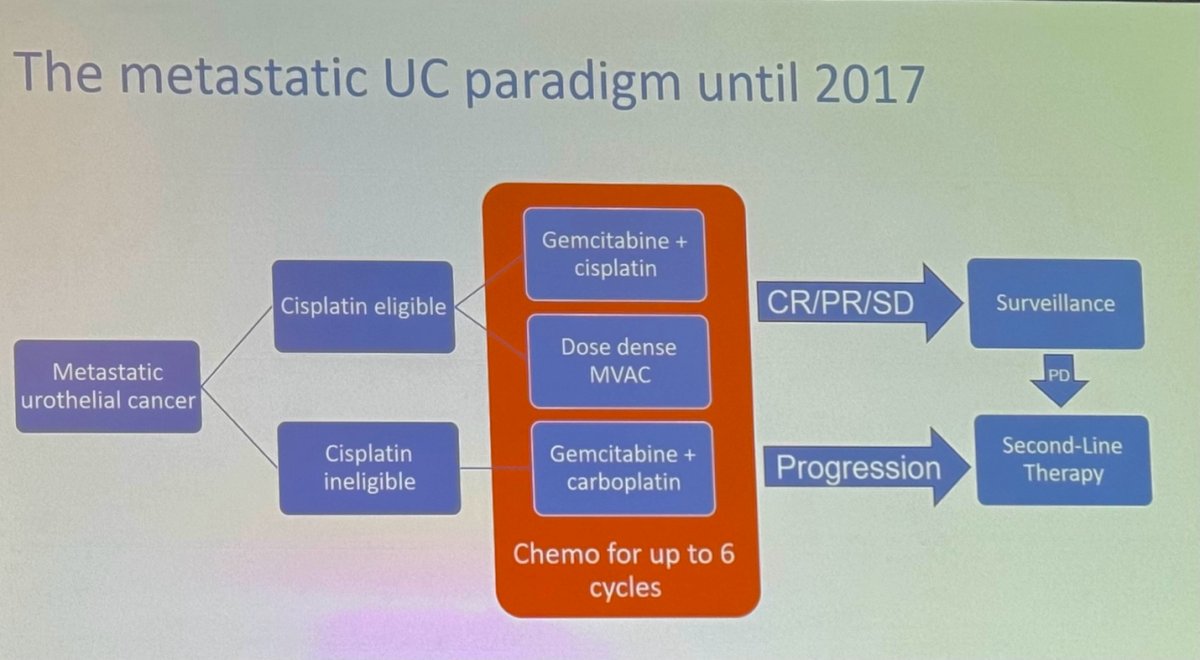
In 2017, Atezolizumab (anti-PD-L1) and Pembrolizumab (anti-PD-1) were both studied as first-line treatments for cisplatin-ineligible patients with locally advanced or metastatic urothelial carcinoma (mUC). Atezolizumab was evaluated in a multicenter, two-cohort phase 2 study. Single-agent Atezolizumab showed a median overall survival (OS) of 15.9 months and an objective response rate (ORR) of 23%, which was significantly better compared to the historical results from the Gemcitabine/Carboplatin regimen.4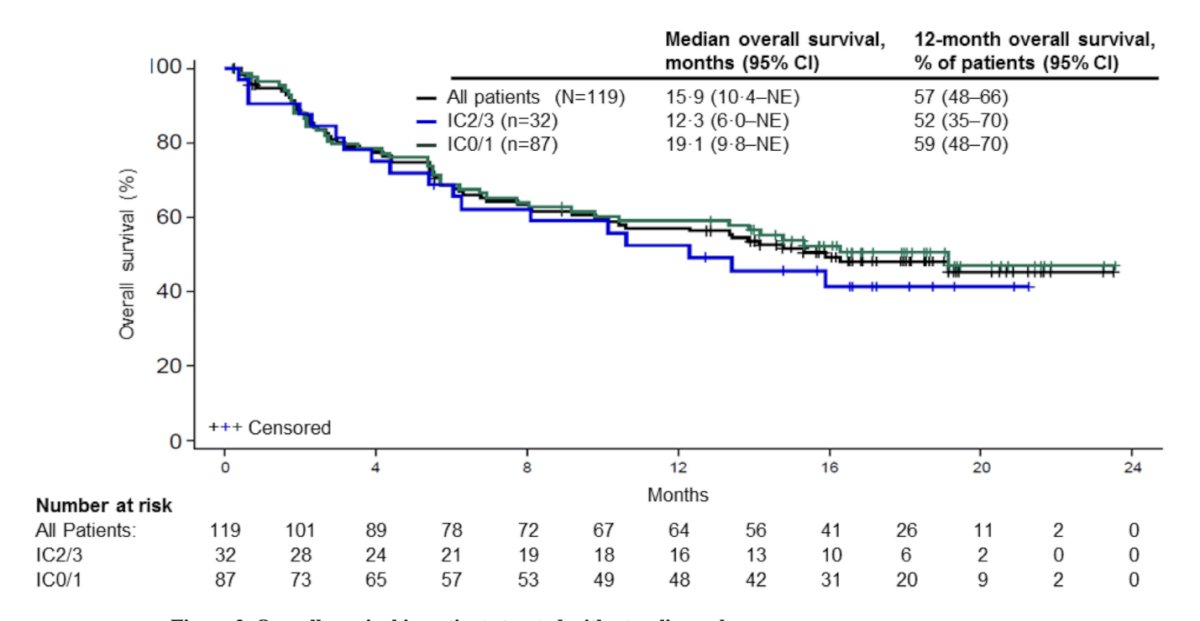
The treatment paradigm for mUC changed with the publication of these studies, and as a result, Atezolizumab and Pembrolizumab were both added as treatment options for cisplatin-ineligible patients with mUC.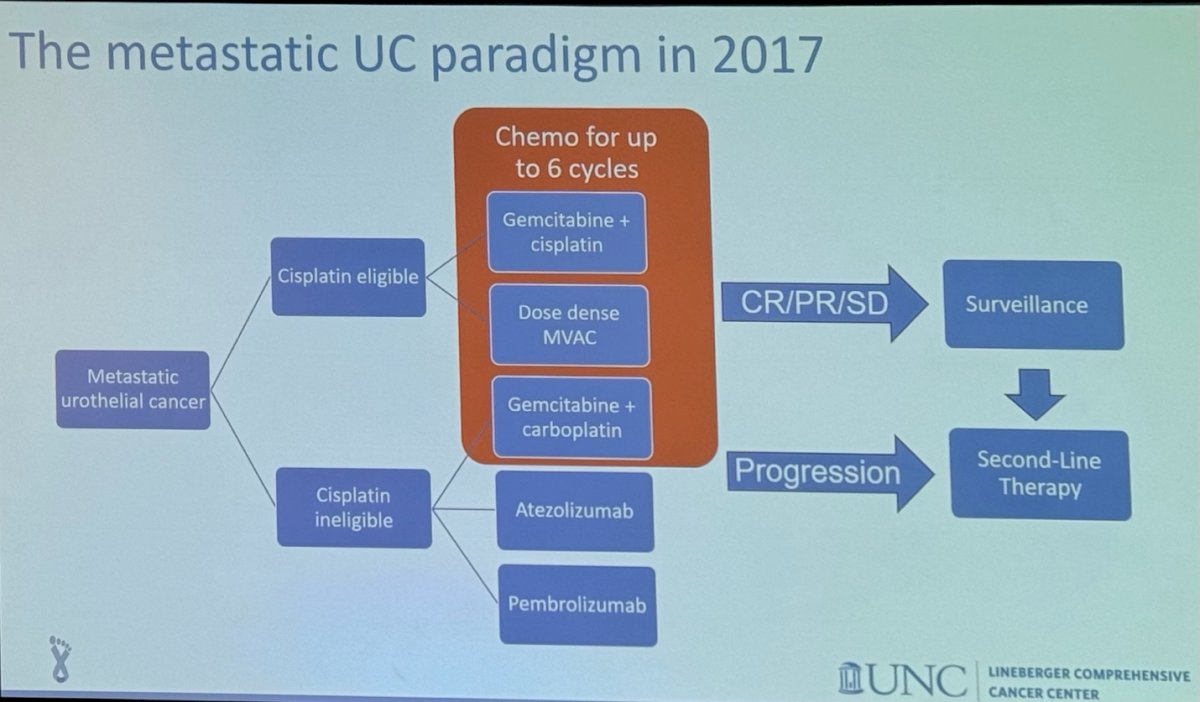
In 2020, the phase 3 JAVELIN Bladder 100 study evaluated Avelumab, an anti-PD-L1, in patients with unresectable LA/mUC who had not experienced disease progression after first-line chemotherapy (gemcitabine plus cisplatin or carboplatin for four to six cycles). Patients were randomized to receive either best supportive care alone or with maintenance Avelumab. The median OS with Avelumab was 21.4 months, compared to 14.3 months with best supportive care alone, reducing the hazard of mortality by 31%. (5) The results of this study changed again the treatment paradigm of mUC.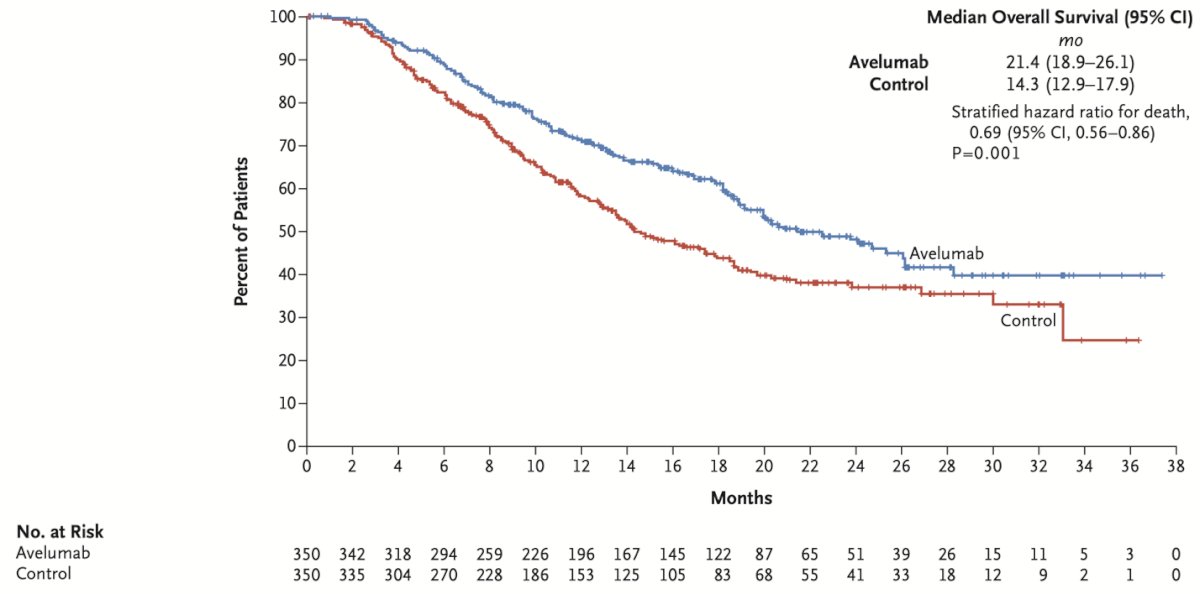
In 2018, the FDA issued a warning about the potential decreased survival in treatment-naïve mUC patients with low PD-L1 expression when treated with Pembrolizumab or Atezolizumab as monotherapy. As a result, clinical guidelines were revised to recommend Atezolizumab and Pembrolizumab in the first-line setting for cisplatin-ineligible mUC patients only if their tumors express PD-L1.
The treatment paradigm for mUC continued to evolve in 2020, becoming more complex but also offering more options for patients.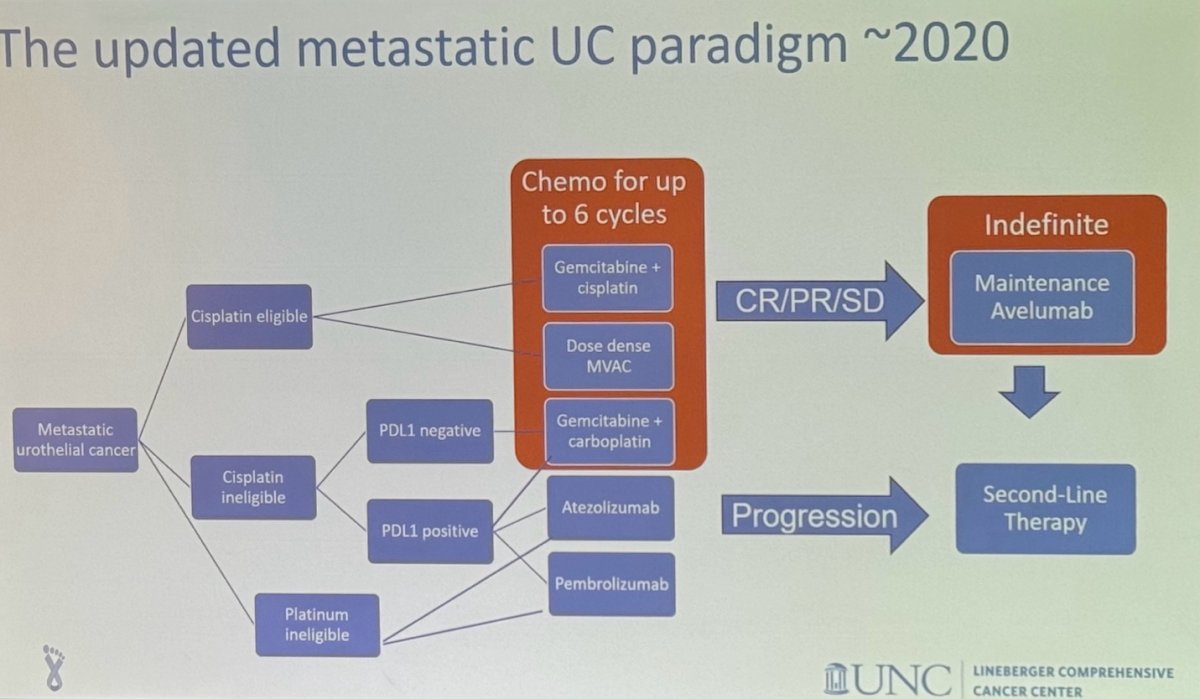
This prompted the development of several phase 3 trials comparing platinum-based chemotherapy (with or without immunotherapy) to immunotherapy alone. Notably, three out of four of these trials were negative, but the CheckMate 901 trial, evaluating the combination of Gem/Cis with Nivolumab, showed positive results with the combination.6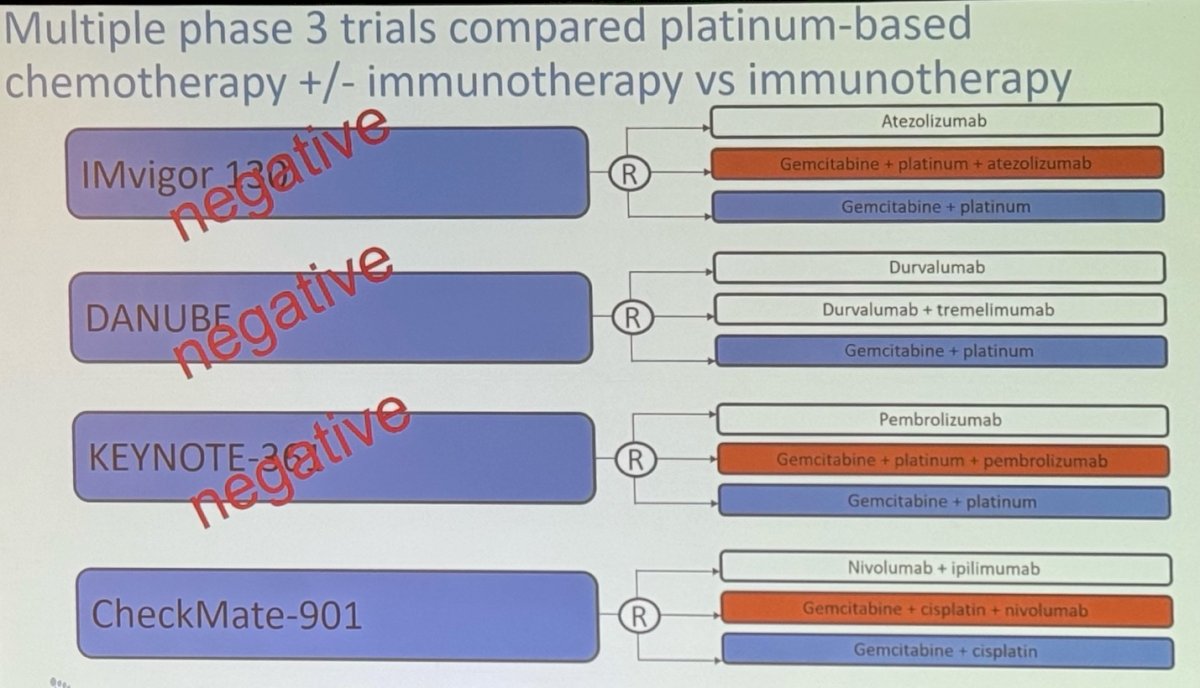
Dr. Rose discussed the CheckMate 901, a phase 3, multinational, open-label study that randomized treatment-naïve patients with unresectable/mUC to receive nivolumab (360 mg) plus gemcitabine–cisplatin for up to six cycles, followed by nivolumab for a maximum of 2 years, or gemcitabine–cisplatin alone for six cycles.
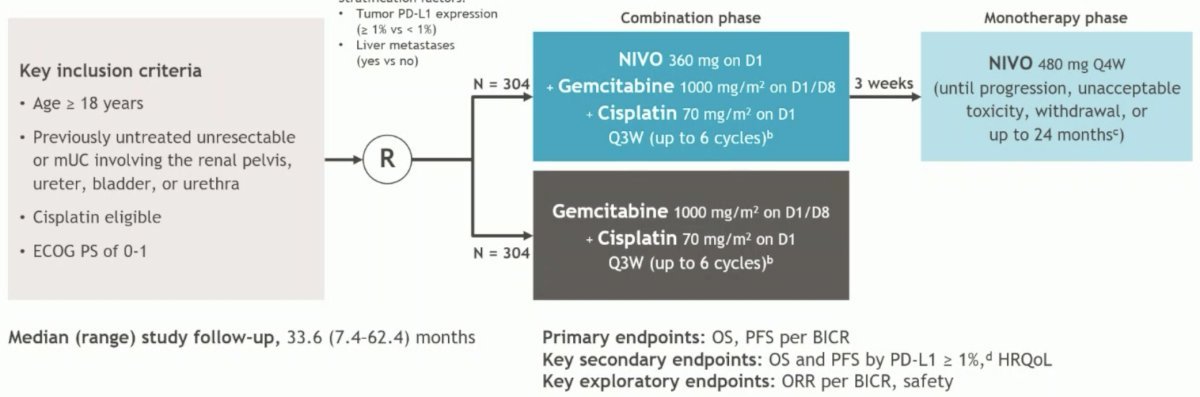
CheckMate 901 demonstrated an impressive ORR of 57.6% and a median OS of 21.7 months in the combination arms, reducing the risk of mortality by 22% compared to gemcitabine/cisplatin, and it was poised to become the next standard of care in the first-line setting for mUC.6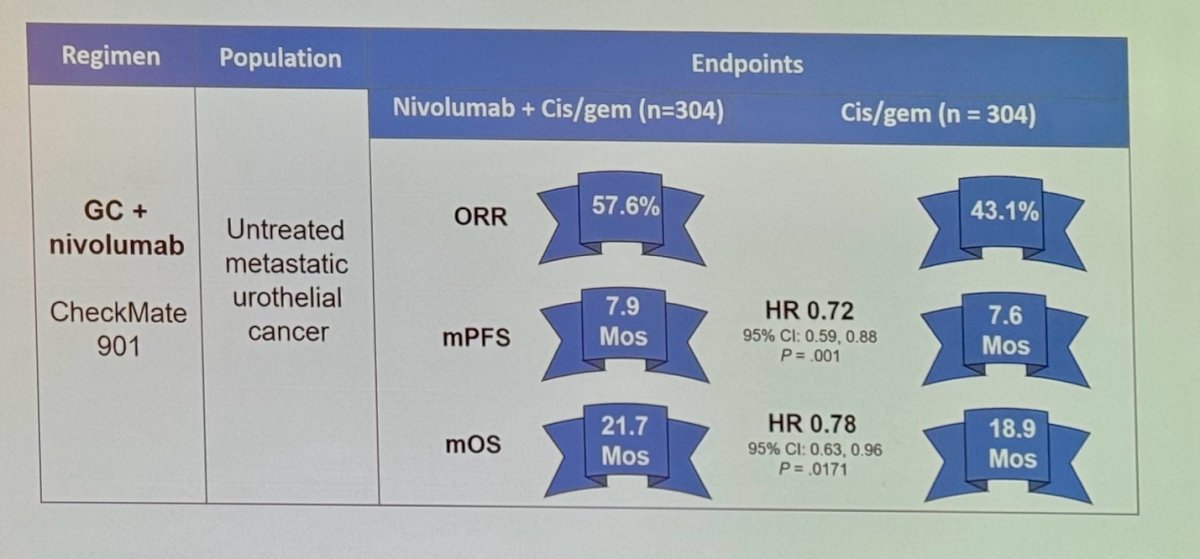
However, with the revolution of antibody-drug conjugates (ADCs), the treatment landscape of mUC has progressed even further. ADCs are monoclonal antibodies linked to a cytotoxic payload (chemotherapy) that bind to specific targets, causing DNA or microtubule disruption and ultimately leading to cell death.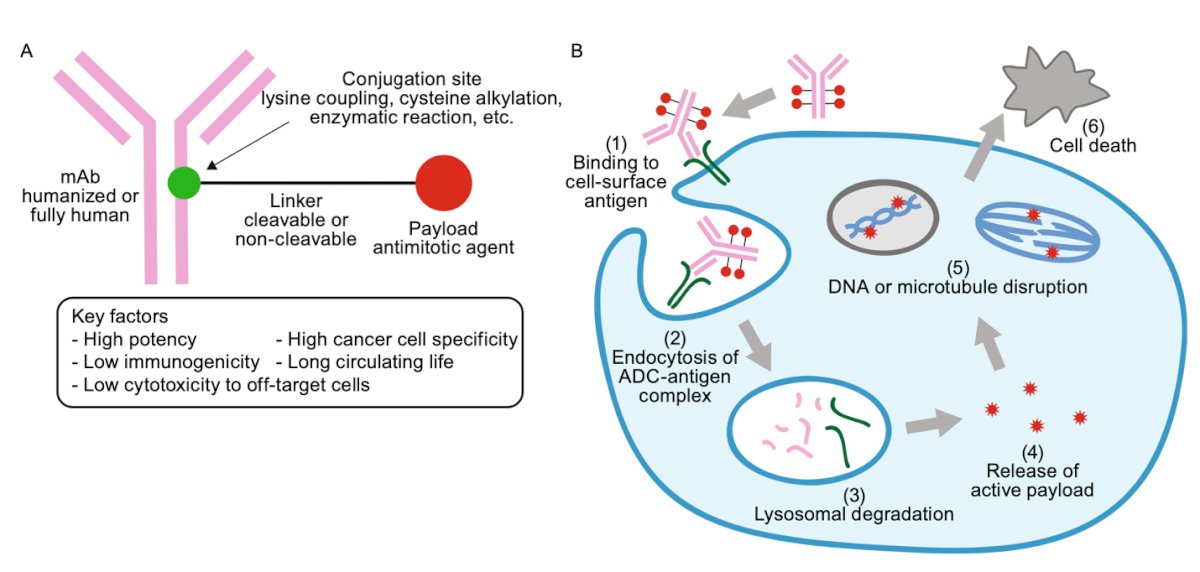
Enfortumab vedotin (EV), an ADC targeting Nectin-4, first demonstrated efficacy in the EV-103 Cohort K study. This Phase 3 study evaluated EV in combination with pembrolizumab versus chemotherapy as a first-line treatment for locally advanced or metastatic urothelial carcinoma (la/mUC), regardless of cisplatin eligibility. EV + pembrolizumab showed high response rates independent of PD-L1 status, as shown in the figure below: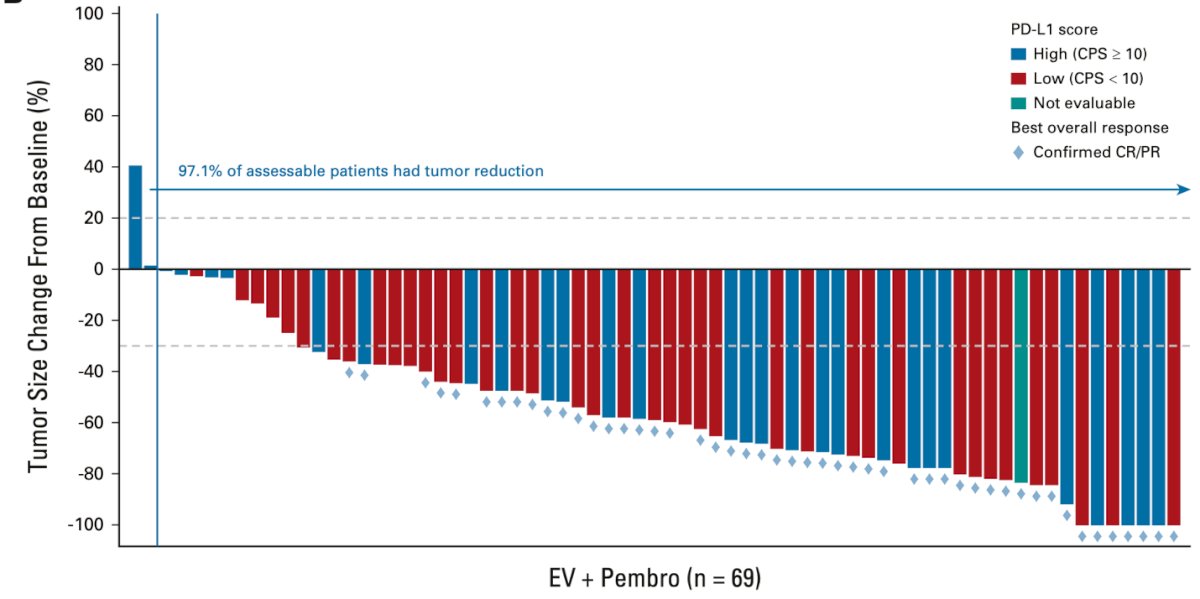
This led to the FDA granting accelerated approval to EV with pembrolizumab for locally advanced or metastatic urothelial carcinoma on April 3, 2023. After this the Landmark EV-302 study was published in 2024, this study randomized patients with treatment naïve mUC to EV + Pembrolizumab vs. Gemcitabine+platinum (cisplatin/carboplatin).
The overall survival subgroup analyses in favor of enfortumab vedotin + pembrolizumab, with clinically meaningful HR and median survival improvements across all subgroup comparisons.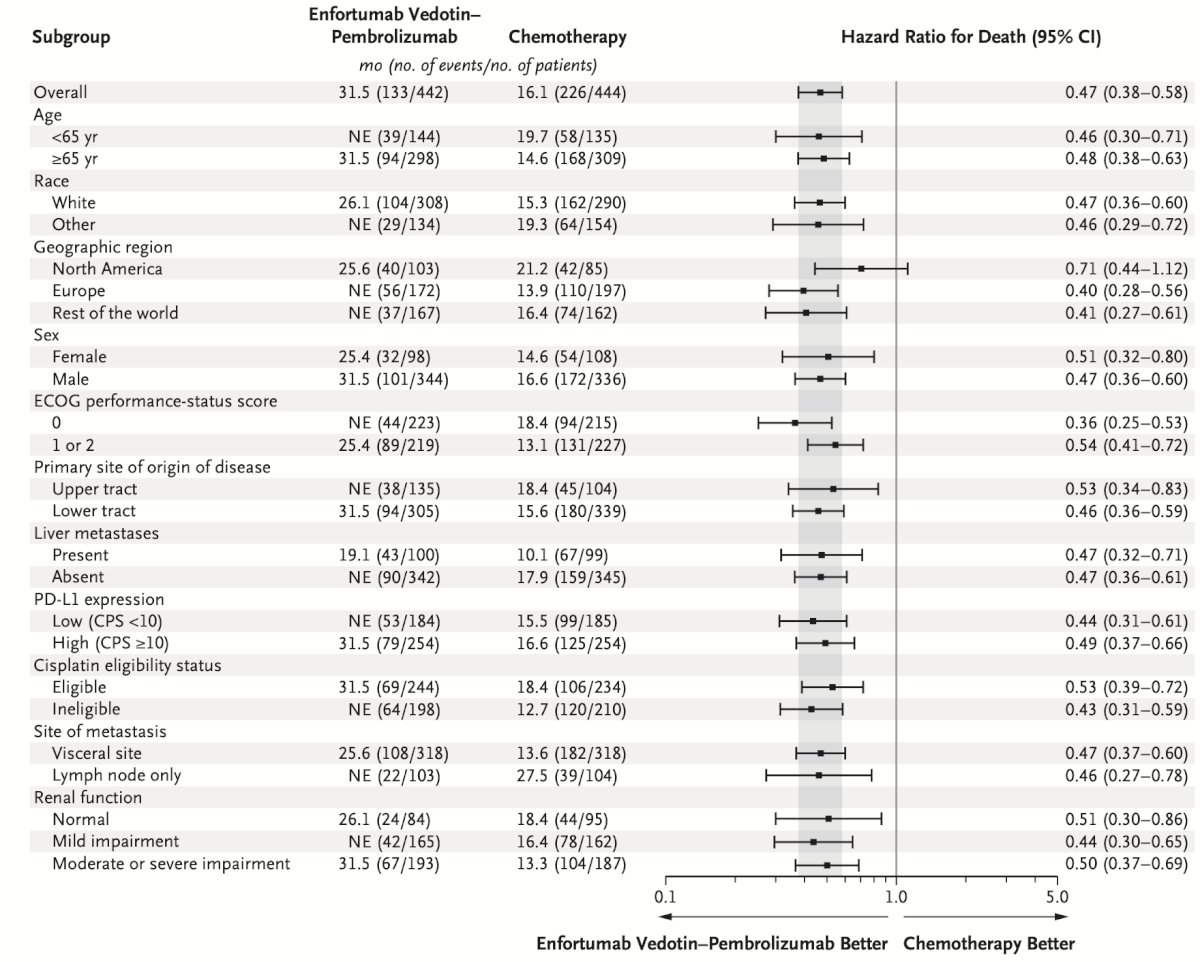
The median OS was 31.5 months in the EV-pembrolizumab group compared to 16.1 months in the chemotherapy group, representing a 53% lower risk of death in the enfortumab vedotin-pembrolizumab group than in the chemotherapy group.8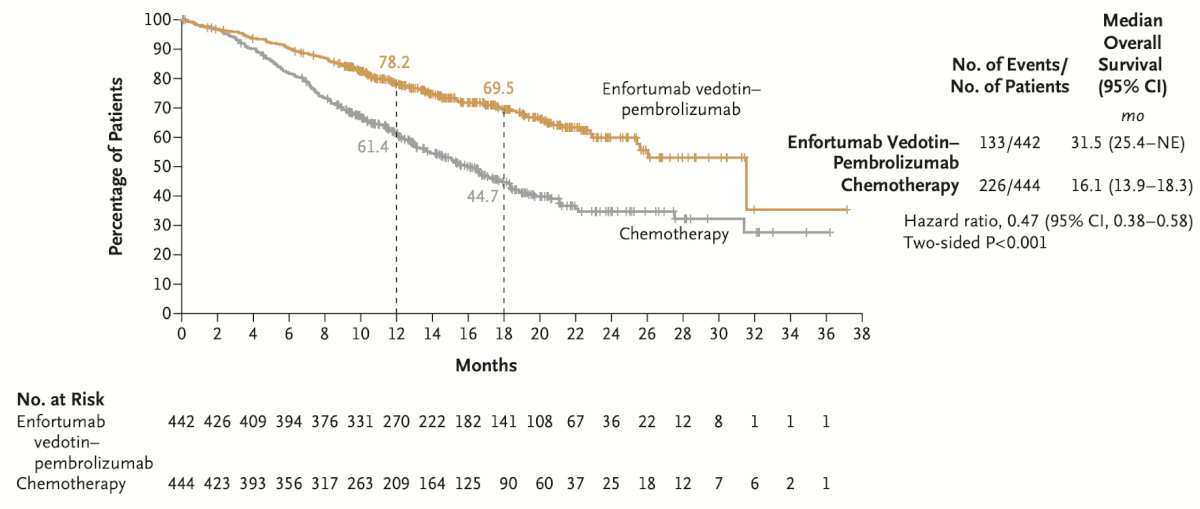
The ORR of EV+ Pembrolizumab was 68%, exceeding all previously reported ORR in any study in the first line setting for the treatment of mUC.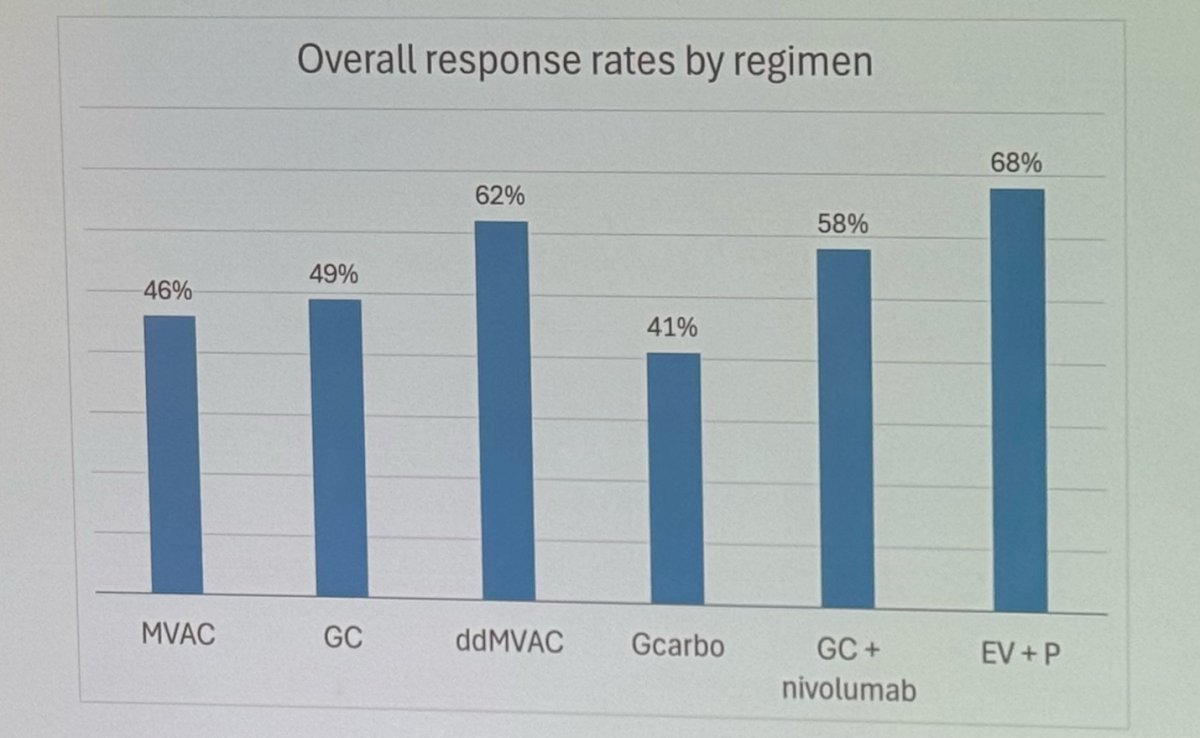
The median OS was doubled with EV+Pembrolizumab compared to any platinum-based chemotherapy regimen.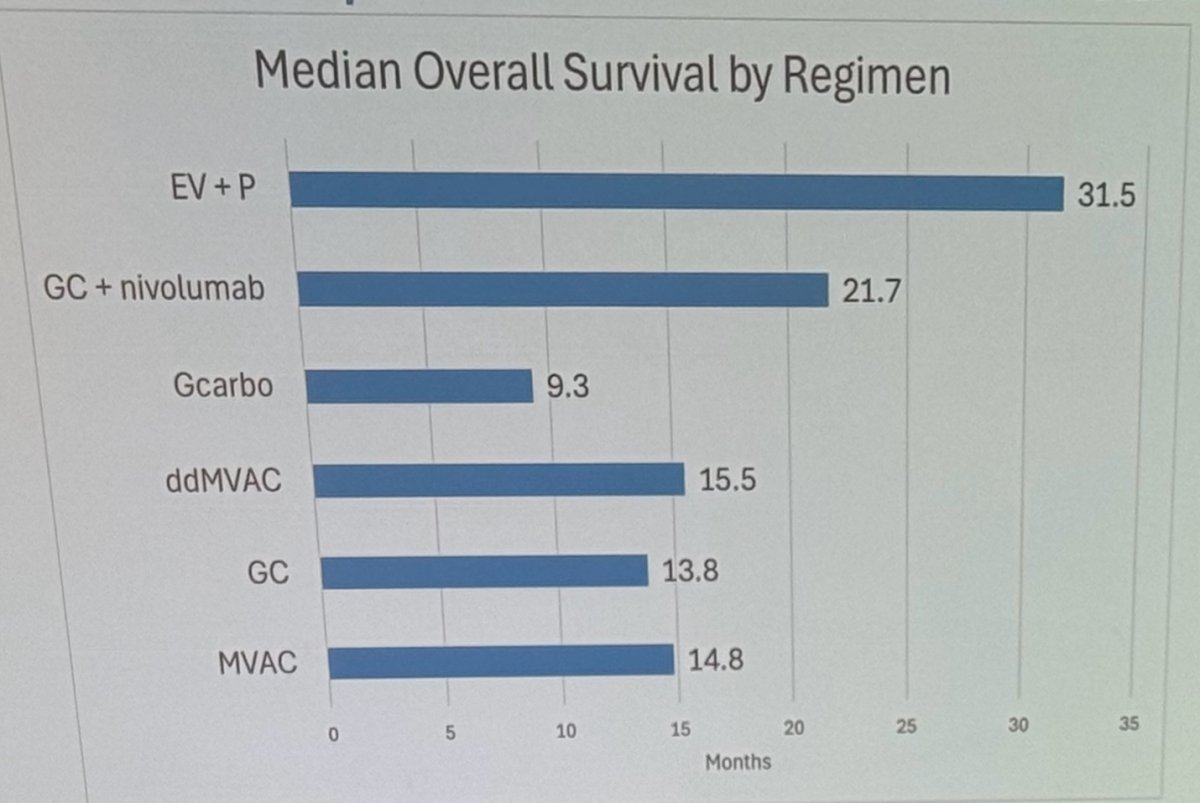
Importantly, the 5-year follow-up results of the initial EV-103 Cohort A (dose escalation), presented at ESMO this year, showed that 47% of responders maintained their response at 5 years, and 41.5% of all patients were alive at 5 years.
AEs associated with EV + Pembrolizumab can be attributed to the individual drugs or both. Pembrolizumab may cause thyroid disorders, arthritis, and hepatitis, while EV is linked to ocular disorders, neuropathy, and hyperglycemia. Common AEs like rash, pneumonitis, and diarrhea can be related to both therapies. While these AEs are manageable, their toxicity profiles should be closely monitored, with appropriate interventions as needed.
The treatment paradigm of mUC has evolved again in 2024 (Shown below). The 2024 EAU guidelines for mUC treatment recommend a simplified approach: patients eligible for combination therapy (EV + P) should receive this regimen. For patients not eligible for combination therapy but who are cisplatin-eligible, platinum/gemcitabine should be used. If patients are cisplatin-ineligible and PD-L1 positive, the preferred options are atezolizumab or pembrolizumab.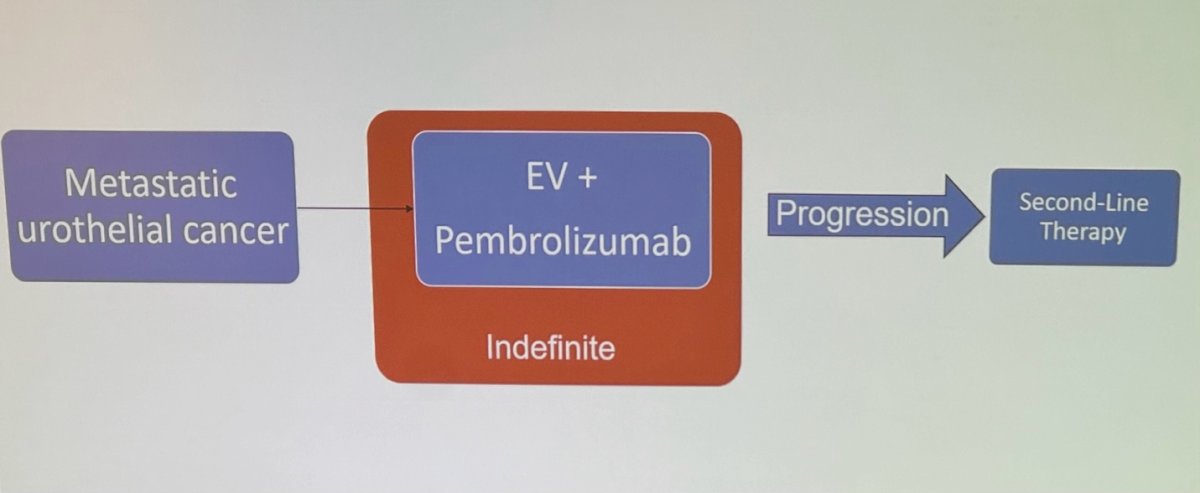
Dr. Rose's comments on EV + Pembrolizumab eligibility are supported by the findings from the EV-302 study, which excluded patients with poorly controlled diabetes (HbA1c ≥ 8.0), grade 2 or higher neuropathy, and those with recent active autoimmune diseases requiring systemic therapy. The subgroup analysis indicated that all patient subgroups, including older patients, those with upper tract primary tumors, and cisplatin-ineligible patients, benefitted from the EV + Pembrolizumab combination.
Dr. Rose concluded her presentation with the following key messages:
- Enfortumab vedotin + pembrolizumab is the treatment of choice for metastatic urothelial cancer
- Overall survival for metastatic UC has doubled with EV + Pembrolizumab compared with platinum-based chemotherapy
- There are very few patients that are not eligible for EV + Pembrolizumab. However, the availability worldwide may influence treatment decision-making
Presented by: Tracy Rose, MD, MPH, Medical Oncologist at the University of North Carolina School of Medicine. Chapel Hill, NC, United States of America.
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, between the 3rd and 6th of December, 2024.
References:- von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A, Lippert CM, Kerbrat P, Sanchez Rovira P, Wersall P, Cleall SP, Roychowdhury DF, Tomlin I, Visseren-Grul CM, Conte PF. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000 Sep;18(17):3068-77. doi: 10.1200/JCO.2000.18.17.3068. PMID: 11001674.
- Sternberg CN, de Mulder PH, Schornagel JH, Théodore C, Fossa SD, van Oosterom AT, Witjes F, Spina M, van Groeningen CJ, de Balincourt C, Collette L; European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group. Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. J Clin Oncol. 2001 May 15;19(10):2638-46. doi: 10.1200/JCO.2001.19.10.2638. PMID: 11352955.
- De Santis M, Bellmunt J, Mead G, Kerst JM, Leahy M, Maroto P, Gil T, Marreaud S, Daugaard G, Skoneczna I, Collette S, Lorent J, de Wit R, Sylvester R. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. 2012 Jan 10;30(2):191-9. doi: 10.1200/JCO.2011.37.3571. Epub 2011 Dec 12. PMID: 22162575; PMCID: PMC3255563.
- Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, Loriot Y, Necchi A, Hoffman-Censits J, Perez-Gracia JL, Dawson NA, van der Heijden MS, Dreicer R, Srinivas S, Retz MM, Joseph RW, Drakaki A, Vaishampayan UN, Sridhar SS, Quinn DI, Durán I, Shaffer DR, Eigl BJ, Grivas PD, Yu EY, Li S, Kadel EE 3rd, Boyd Z, Bourgon R, Hegde PS, Mariathasan S, Thåström A, Abidoye OO, Fine GD, Bajorin DF; IMvigor210 Study Group. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017 Jan 7;389(10064):67-76. doi: 10.1016/S0140-6736(16)32455-2. Epub 2016 Dec 8. Erratum in: Lancet. 2017 Aug 26;390(10097):848. doi: 10.1016/S0140-6736(17)32213-4. PMID: 27939400; PMCID: PMC5568632.
- Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, Kalofonos H, Radulović S, Demey W, Ullén A, Loriot Y, Sridhar SS, Tsuchiya N, Kopyltsov E, Sternberg CN, Bellmunt J, Aragon-Ching JB, Petrylak DP, Laliberte R, Wang J, Huang B, Davis C, Fowst C, Costa N, Blake-Haskins JA, di Pietro A, Grivas P. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med. 2020 Sep 24;383(13):1218-1230. doi: 10.1056/NEJMoa2002788. Epub 2020 Sep 18. PMID: 32945632.
- van der Heijden MS, Sonpavde G, Powles T, Necchi A, Burotto M, Schenker M, Sade JP, Bamias A, Beuzeboc P, Bedke J, Oldenburg J, Chatta G, Ürün Y, Ye D, He Z, Valderrama BP, Ku JH, Tomita Y, Filian J, Wang L, Purcea D, Patel MY, Nasroulah F, Galsky MD; CheckMate 901 Trial Investigators. Nivolumab plus Gemcitabine-Cisplatin in Advanced Urothelial Carcinoma. N Engl J Med. 2023 Nov 9;389(19):1778-1789. doi: 10.1056/NEJMoa2309863. Epub 2023 Oct 22. PMID: 37870949.
- O'Donnell PH, Milowsky MI, Petrylak DP, Hoimes CJ, Flaig TW, Mar N, Moon HH, Friedlander TW, McKay RR, Bilen MA, Srinivas S, Burgess EF, Ramamurthy C, George S, Geynisman DM, Bracarda S, Borchiellini D, Geoffrois L, Maroto Rey JP, Ferrario C, Carret AS, Yu Y, Guseva M, Homet Moreno B, Rosenberg JE. Enfortumab Vedotin With or Without Pembrolizumab in Cisplatin-Ineligible Patients With Previously Untreated Locally Advanced or Metastatic Urothelial Cancer. J Clin Oncol. 2023 Sep 1;41(25):4107-4117. doi: 10.1200/JCO.22.02887. Epub 2023 Jun 27. PMID: 37369081; PMCID: PMC10852367.
- Powles T, Valderrama BP, Gupta S, Bedke J, Kikuchi E, Hoffman-Censits J, Iyer G, Vulsteke C, Park SH, Shin SJ, Castellano D, Fornarini G, Li JR, Gümüş M, Mar N, Loriot Y, Fléchon A, Duran I, Drakaki A, Narayanan S, Yu X, Gorla S, Homet Moreno B, van der Heijden MS; EV-302 Trial Investigators. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N Engl J Med. 2024 Mar 7;390(10):875-888. doi: 10.1056/NEJMoa2312117. PMID: 38446675.


