(UroToday.com) The 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX was host to an Advanced Disease and Adjuvant Therapy session. Dr. Omar Mian discussed the role of adjuvant radiotherapy in the post-radical cystectomy setting, addressing the following points:
- Why consider adjuvant radiotherapy after cystectomy?
- Peri-operative systemic therapy and local control
- Evidence for the efficacy of adjuvant radiotherapy
- Evidence for the safety and tolerability of adjuvant radiotherapy
- Emerging paradigms and future directions
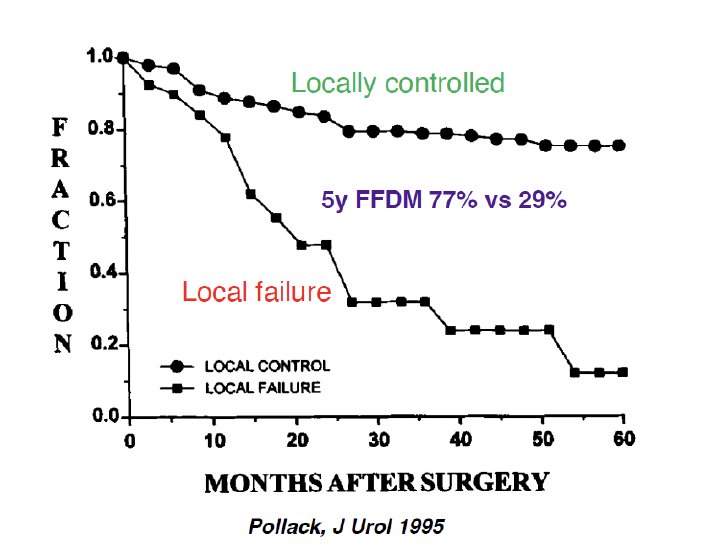
It has long been established that local control correlates with distant metastasis in patients with muscle invasive bladder cancer. In a retrospective series of 240 patients treated with radical cystectomy +/- multiagent chemotherapy at The MD Anderson Cancer Center between 1984 and 1990 for cT2-4 transitional cell carcinoma of the bladder, Pollack et al. demonstrated that the actuarial 5-year freedom from distant metastasis rate for those with local control was 77%, compared to 29% for those with local failure (p < 0.0001). Significantly, patients with locally advanced disease (i.e., pT3-4, pN+, positive margins, lymphovascular invasion, <10 lymph nodes removed) were found to be at higher risk of locoregional failure as the initial site of relapse.1
Numerous models/nomograms have been since developed to predict the risk of locoregional failure using clinicopathologic variables and help guide the use of adjuvant radiotherapy and/or chemotherapy. Using the patient cohort of SWOG 8710, a randomized trial of radical cystectomy +/- chemotherapy, Christodouleas et al. developed a revised stratification model using pT classification, margin status, and the number of lymph nodes to stratify patients into 1 of 3 risk subgroups of increasing locoregional failure risk:2
- Low risk (≤pT2): Predicted 5-year locoregional failure risk of 8%
- Intermediate risk (≥pT3 with negative margins and ≥10 lymph nodes identified): 20% risk
- High risk (≥pT3 with positive margins OR <10 lymph nodes identified): 41% risk

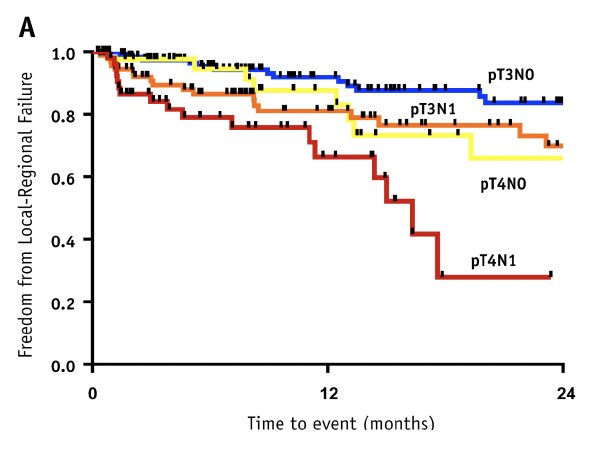
Even among patients with higher risk pathologic features (i.e., pT3-4N0-1), further risk stratification to predict those at higher risk of locoregional failure can be performed to help guide the appropriate use of adjuvant radiation therapy. In a multi-institutional series of 334 patients with pT3-4 and N0-1 disease after radical cystectomy, of whom 46% received peri-operative chemotherapy, it was demonstrated that patients with pT3N0 disease have a 2-year incidence of locoregional failure of 12%. Comparatively, intermediate (pT3N1 or pT4N0) and high-risk patients (pT4N1) had 2-year rates of 33% and 72%, respectively.3
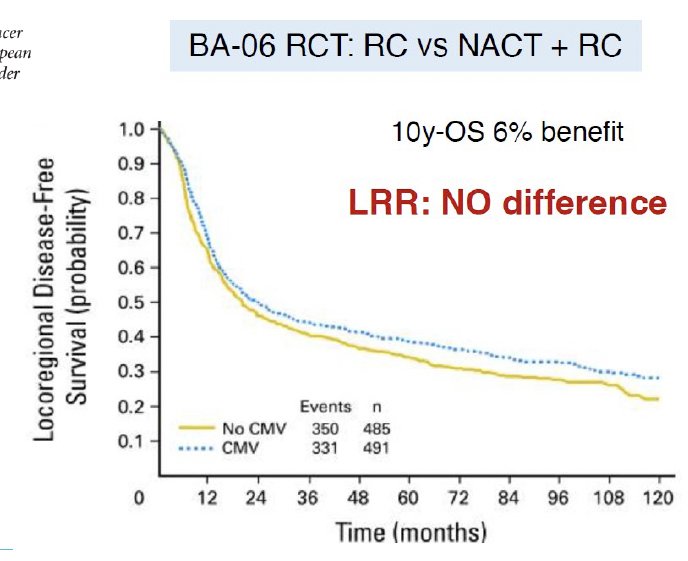
An important clinical rationale for the use of adjuvant pelvic radiotherapy is the fact that peri-operative chemotherapy and immunotherapy have been shown to reduce the rates of distant disease development, but not the rates of local-regional relapse in muscle-invasive bladder cancer. The phase III BA06 30894 trial of neoadjuvant cisplatin + methotrexate + vinblastine (CMV) failed to demonstrate a locoregional relapse benefit (HR: 0.96, p=0.63), despite being associated with a 6% 10-year overall survival benefit.4
While a disease-free survival benefit was demonstrated in two trials of adjuvant immunotherapy, namely CheckMate 274 (nivolumab)5 and AMBASSADOR (pembrolizumab),6 the impact of these drugs on subsequent locoregional control has not been reported.
Another strong rationale for the use of adjuvant radiotherapy for muscle invasive bladder cancer patients in the post-radical cystectomy setting is the fact that local failures in the pelvis after cystectomy are rarely salvageable due to the difficulty of delivering definitive dose radiation (≥60Gy). This was demonstrated in 2013 by Baumann et al., who published a series of 442 patients who underwent a radical cystectomy plus pelvic lymph node dissection, with or without chemotherapy, for localized urothelial cancer. As expected, the 5-year overall survival rates for ≥pT3 and ≤pT2 patients were 22% and 60%, respectively. Significantly, of the 80 patients with local failure, only one survived >5 years.7
Overall, the rationale for adjuvant radiation therapy is supported by the following:
- Local failure metastasis, morbidity, mortality
- High risk of locoregional recurrence in patients with locally advanced disease (i.e., ≥pT3, pN+, and positive surgical margins)
- Systemic therapy improves survival outcomes but is not as effective for local control
- Salvage therapy for established local failure is challenging
Next, Dr. Mian addressed the following questions:
- Does adjuvant radiotherapy improve outcomes
- Is adjuvant radiotherapy safe/tolerable?
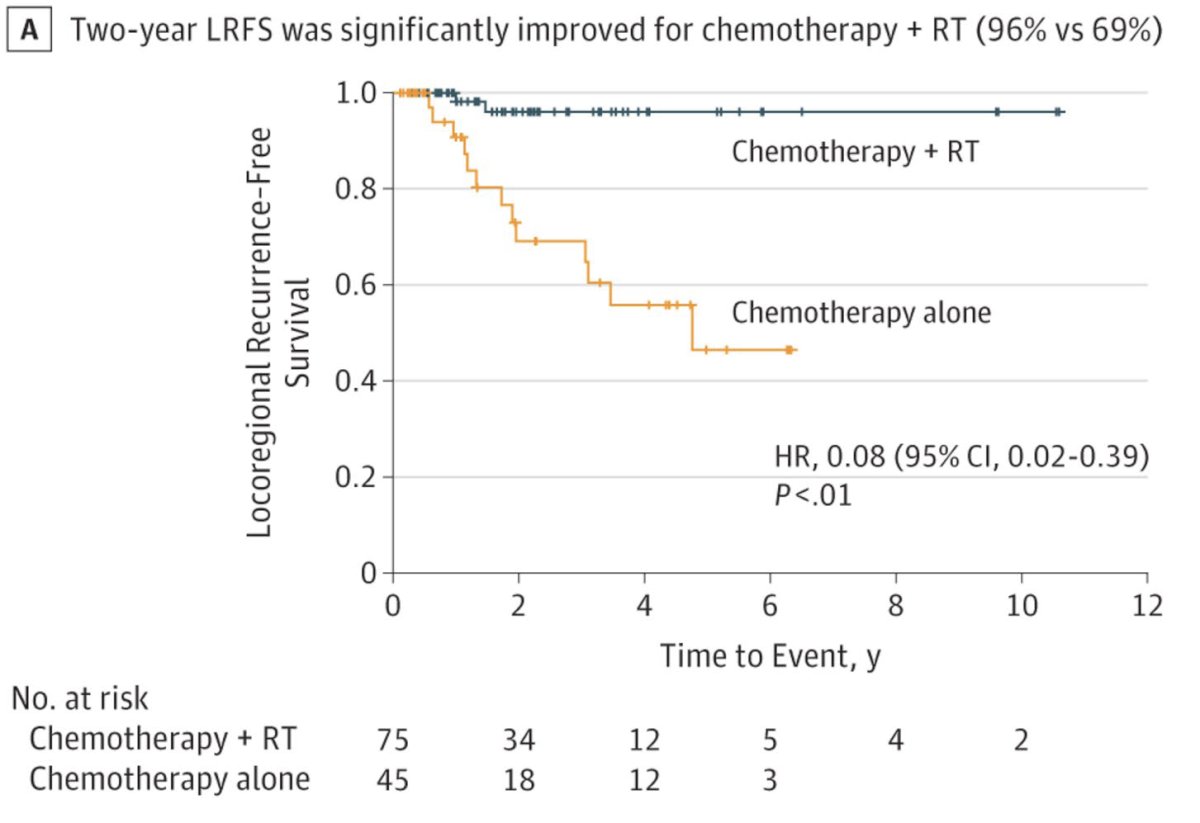
In 2018, the results of a randomized phase II trial comparing adjuvant sequential radiotherapy plus chemotherapy to adjuvant chemotherapy alone for patients with locally advanced bladder cancer were published. Eligible patients had pT3-4N0-1disease with negative surgical margins. Adjuvant chemotherapy entailed 4 cycles of gemcitabine + cisplatin. Radiotherapy was administered at a dose of 4500 cGy in 150 cGy twice-daily fractions over 3 weeks using 3-dimensional conformal techniques.
The patients in the combination radiotherapy + chemotherapy arm had superior two-year locoregional recurrence-free survival rates (96% versus 69%; HR: 0.08, p<0.01). Similarly, the disease-free survival (68% vs 56%, p=0.07) and overall survival rates (71% vs 60%, p=0.11) favored the chemotherapy + radiotherapy arm.8
From a safety profile standpoint, 6.7% of patients in the chemotherapy + radiotherapy arm experienced late grade ≥3 gastrointestinal adverse events, compared to 2.2% of those in the chemotherapy alone arm.8

The safety profile of adjuvant radiotherapy can be further improved by the incorporation of novel targeting techniques, including intensity modulated radiotherapy (IMRT), which can more effectively limit radiation to the bowel in the central pelvis and spare the urinary diversion.
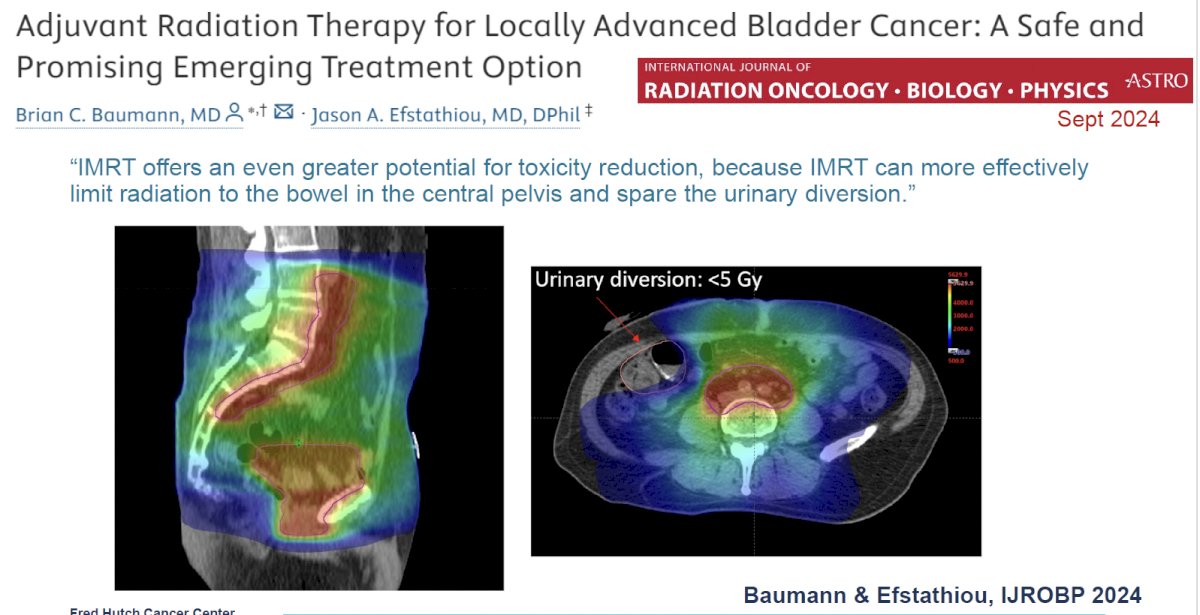
In a single arm study performed at Ghent University Hospital in Belgium, 72 patients with high-risk features after radical cystectomy (≥pT3 ± LVI, <10 LNs removed, pN1, or positive margins) underwent adjuvant IMRT (50 Gy in 25 fractions to the pelvic nodes with inclusion of the cystectomy bed for positive margins). Of these 72 patients, five (7%) developed acute grade ≥3 gastrointestinal toxicities.9
The safety and efficacy of adjuvant radiation therapy following radical cystectomy in locally advanced urothelial carcinoma of the bladder was demonstrated in a recent randomized trial by Zaghloul et al. 122 patients with locally advanced urothelial carcinoma were randomized to receive adjuvant radiotherapy (50 Gy in 25 fractions) 4 weeks after cystectomy versus cystectomy alone. Adjuvant IMRT was administered to the pelvic nodes and cystectomy bed. The 3-year locoregional recurrence-free survival was 81% in the adjuvant radiotherapy arm versus 71% for those in the observation arm (p=0.046).1
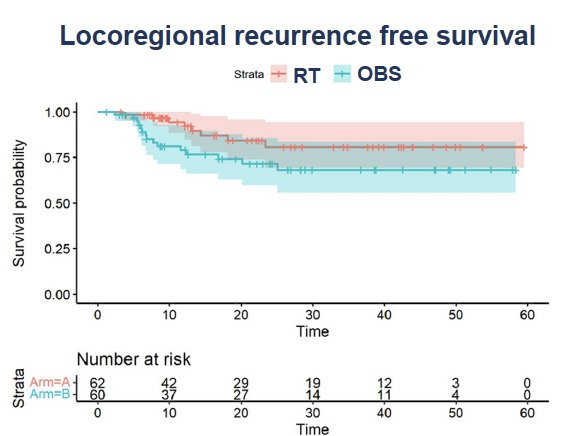
One patient in each arm experienced late grade ≥3 gastrointestinal toxicity, and one patient in the adjuvant radiotherapy arm experienced a grade ≥3 genitourinary adverse event. There were 11 patients with a neobladder (6 in adjuvant and 5 in control arm), of whom 4/11 developed late grade 1–3 genitourinary toxicity (2 in each arm).10

BART (Bladder Adjuvant RadioTherapy) is an Indian multicenter phase III randomized trial that randomized 153 high-risk muscle invasive bladder cancer patients post-radical cystectomy to either adjuvant radiotherapy (50.4 Gy in 28 fractions) or observation. The primary study endpoint was 2-year locoregional failure-free survival. The initial results were presented by Dr. Murthy at the 2024 American Society for Radiation Oncology (ASTRO) annual meeting.
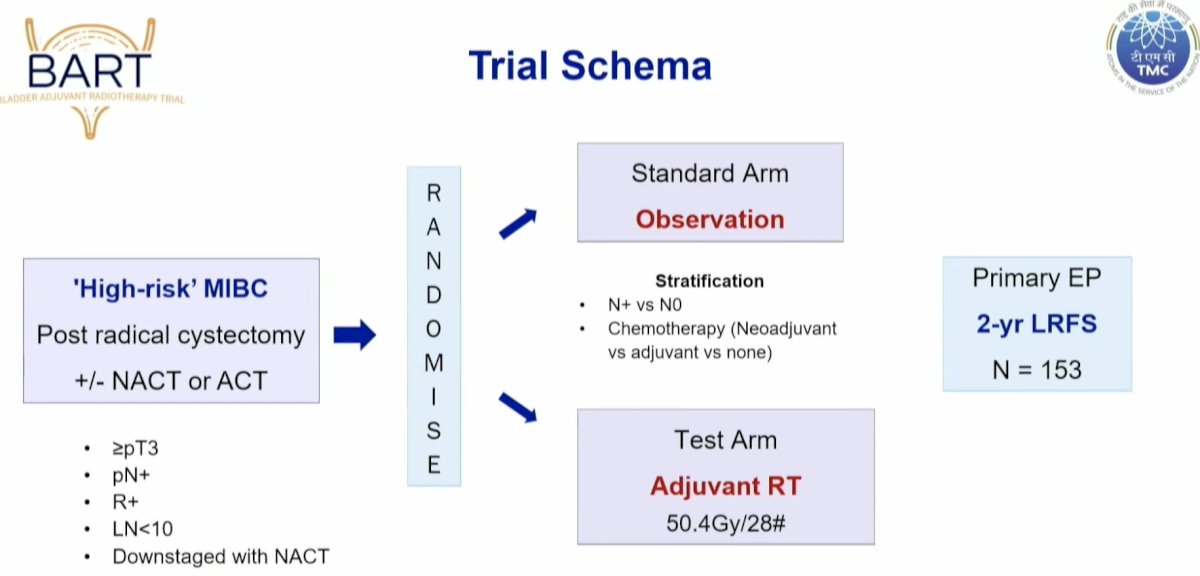
From a technical standpoint, contouring was performed according to the 2016 IJROBP consensus guidelines. The contouring volume was modified to include the cystectomy bed (2 cm above the pubic symphysis to the penile bulb). They also included the common iliac nodes up to the aortic bifurcation in the radiotherapy clinical target volume. The standard ‘organs at risk’ were contoured, including the stoma, anorectum, femurs, and neobladder or ileal conduit, where applicable. All patients were treated with intensity modulated, image-guided radiotherapy.

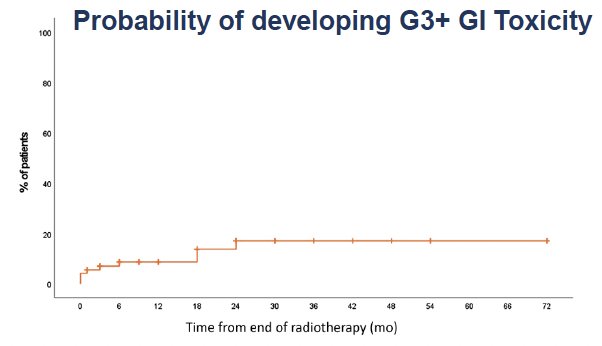
In the acute setting., none of the patients had to discontinue radiotherapy secondary to toxicity. Grade 2 acute toxicity was observed more commonly in the radiotherapy arm (17.5% versus 1.4%) and were most commonly bowel symptoms that self-resolved and did not require surgical intervention or inpatient hospitalization. Grade 3 events were observed less frequently in the radiotherapy arm (1.6% versus 4.1%). Notably, 67% of patients who received radiotherapy experienced no symptoms (red arrow below).
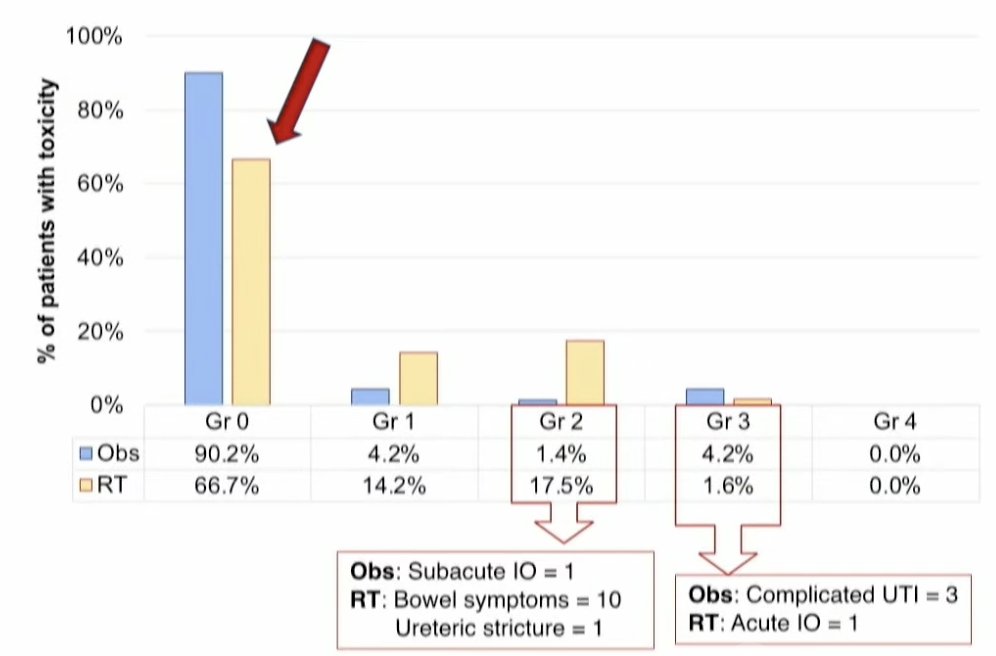
At a median follow-up of 27 months, late grade 2 adverse events were observed in 15% of radiotherapy-treated patients and 7% of patients in the observation arm. Conversely, grade 3/4 adverse events were observed less frequently in the radiotherapy arm (8.4% versus 10.5%).
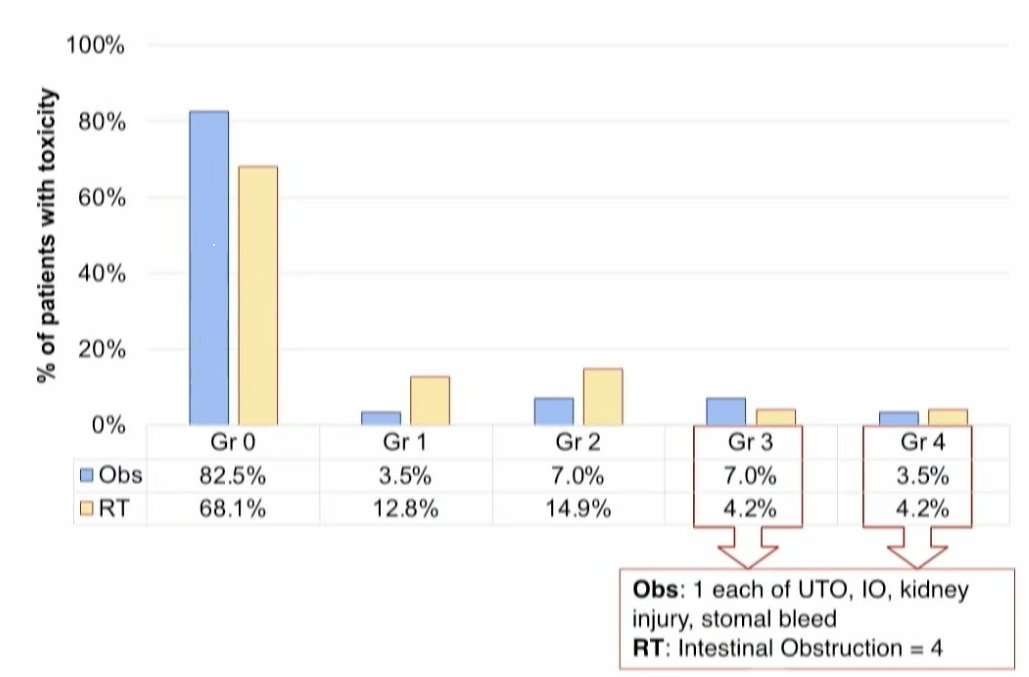
Overall, 23.3% of radiotherapy-treated patients experienced late grade ≥2 toxicity events, compared to 17.5% of patients in the observation arm.

What is the role of adjuvant radiotherapy in the era of peri-operative immunotherapy? The combination of chemo-radiotherapy + immunotherapy (SWOG/NRG 1806: atezolizumab; KEYNOTE 992: pembrolizumab) has demonstrated favorable toxicity profiles in patients with intact muscle invasive bladder cancer. These treatment modalities have non-overlapping toxicities and mechanisms of action, and radiotherapy is known to be immunomodulatory/immunostimulatory. Additionally, adjuvant radiotherapy may be better tolerated than adjuvant immunotherapy, as demonstrated by:
- <10% acute/late grade 3 adverse events in the Ghent, Egyptian, and BART trials
- 18% treatment-related grade 3 adverse events in the CheckMate 274 trial
- 51% overall grade 3 adverse events in the AMBASSADOR trial
As such, adjuvant radiotherapy appears to be a safe and potentially cost-effective option for patients in this setting.
Dr. Mian concluded his presentation as follows:
- Patients with pT3-4, lymphovascular invasion, pN+, and/or positive surgical margins after radical cystectomy +/- neoadjuvant chemotherapy are at greater risk of local failure and related morbidity/mortality
- Modern adjuvant radiation (IMRT) reduces local recurrence risk, improves outcomes, and is generally well-tolerated
- Consider adjuvant treatment, including radiotherapy, for select high-risk patients, per NCCN Guidelines
- Future studies are needed to evaluate the role of radiotherapy combined with emerging systemic treatments in the peri-operative setting
Presented by: Omar Mian, MD, PhD, Associate Professor, Department of Radiation Oncology, University of Washington School of Medicine, Fred Hutch Cancer Center, Seattle, WA
Written by: Rashid K. Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 Society of Urologic Oncology (SUO) Annual Meeting, Dallas, TX, Tues, Dec 3 – Fri, Dec 6, 2024.
References:
- Pollack A, Zagars GK, Cole CJ, et al. The relationship of local control to distant metastasis in muscle invasive bladder cancer. J Urol. 1995; 154(6):2059-63.
- Christodouleas JP, Baumann BC, He J, et al. Optimizing bladder cancer locoregional failure risk stratification after radical cystectomy using SWOG 8710. Cancer. 2014; 120(8):1272-80.
- Reddy AV, Pariser JJ, Pearce SM, et al. Patterns of Failure After Radical Cystectomy for pT3-4 Bladder Cancer: Implications for Adjuvant Radiation Therapy. Int J Radiat Oncol Biol Phys. 2016; 94(5):1031-9.
- IC of Trialists et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011; 29(16):2171-7.
- Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021; 384(22):2102-14.
- Apolo AB, Ballman KV, Sonpavde G, et al. Adjuvant Pembrolizumab versus Observation in Muscle-Invasive Urothelial Carcinoma. N Engl J Med. 2024.
- Baumann BC, Guzzo TJ, He J, et al. Bladder cancer patterns of pelvic failure: implications for adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. 2013; 8592):363-9.
- Zaghloul MS, Christodouleas JP, Smith A, et al. Adjuvant Sandwich Chemotherapy Plus Radiotherapy vs Adjuvant Chemotherapy Alone for Locally Advanced Bladder Cancer After Radical Cystectomy: A Randomized Phase 2 Trial. JAMA Surg. 2018; 153(1):e174591.
- Fonteyne V, Dirix P, Van Praet C, et al. Adjuvant Radiotherapy After Radical Cystectomy for Patients with High-risk Muscle-invasive Bladder Cancer: Results of a Multicentric Phase II Trial. Eur Urol Focus. 2022; 8(5):1238-45.
- Zaghloul MS, Alnagmy AK, Aboul Kasem H, et al. The Value and Safety of Adjuvant Radiation Therapy After Radical Cystectomy in Locally Advanced Urothelial Bladder Cancer: A Controlled Randomized Study. Int J Radiat Oncol Biol Phys. 2024; 120(3):658-66.


