(UroToday.com) The 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX was host to an Advanced Disease and Adjuvant Therapy session. Dr. Jean Hoffman-Censits discussed the current state of adjuvant systemic therapy for urothelial carcinoma.
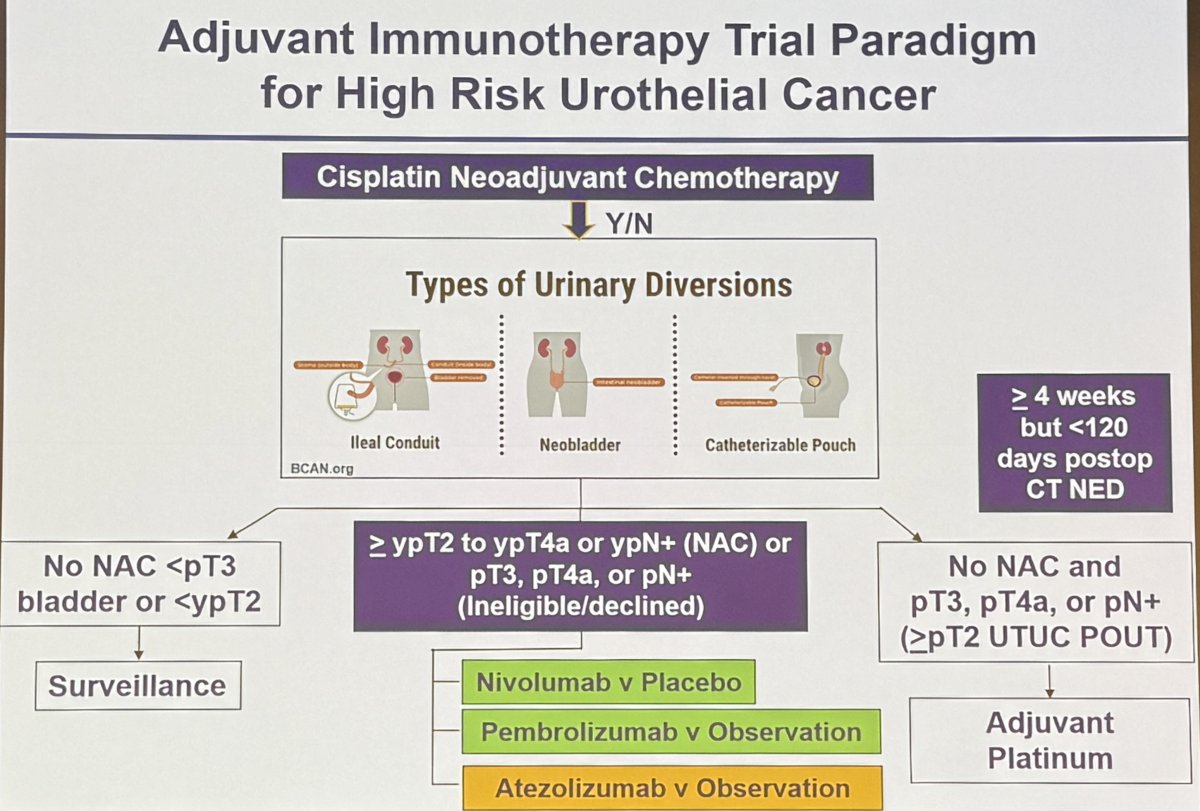
Currently, adjuvant immunotherapy is recommended for patients with high-risk urothelial carcinoma of the upper or lower tracts, with nivolumab FDA-approved in this setting.1 Eligible patients are those meeting the following criteria:
- Neoadjuvant cisplatin pre-treated: ≥ypT2-4a or ypN+
- Declined or not eligible for neoadjuvant cisplatin: pT3-4a or pN+
Dr. Hoffman-Censits noted that the NCCN guidelines statement/section on adjuvant immunotherapy for high-risk urothelial cancer is currently ‘under construction’ and is expected to be available within the next few months.

Summarized below is the current treatment paradigm for adjuvant immunotherapy in high-risk urothelial carcinoma.
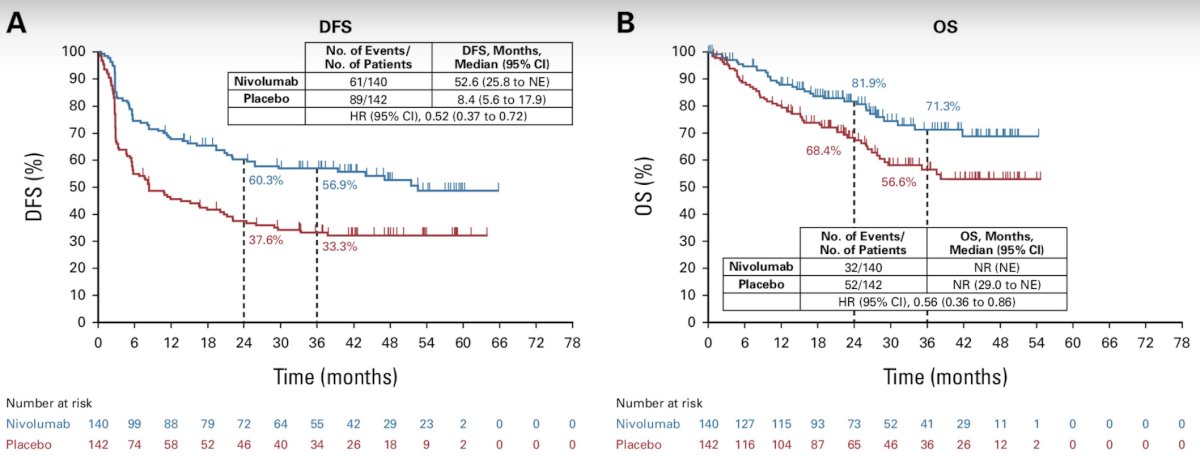
Going into more detail, Dr. Hoffman-Censits next discussed the three trials of adjuvant immunotherapy for high-risk urothelial carcinoma. CheckMate 274 was a phase III, multicenter, double-blind randomized control trial that randomized patients with high-risk, muscle-invasive urothelial carcinoma who had undergone radical surgery to receive, in a 1:1 ratio, either nivolumab (240 mg intravenously) or placebo every 2 weeks for up to 1 year. At a median follow-up of 20.9 months, adjuvant nivolumab was associated with a significant improvement in disease-free survival (median: 20.8 versus 10.8 months).2 Extended follow-up of 36.1 months, demonstrated a continued disease-free survival benefit for nivolumab (median: 22 versus 10.9 months). Importantly, an overall survival benefit was also observed in this updated report, with median survivals of 69.5 versus 50.1 months (HR: 0.76, 95% CI: 0.61–0.96).3
AMBASSADOR was a randomized phase III trial of pembrolizumab versus observation for one year in high-risk metastatic urothelial carcinoma patients who had undergone radical surgery. Published recently in The New England Journal of Medicine, adjuvant pembrolizumab was associated with a median disease-free survival improvement from 14.2 to 29.6 months (HR: 0.73, p=0.003).4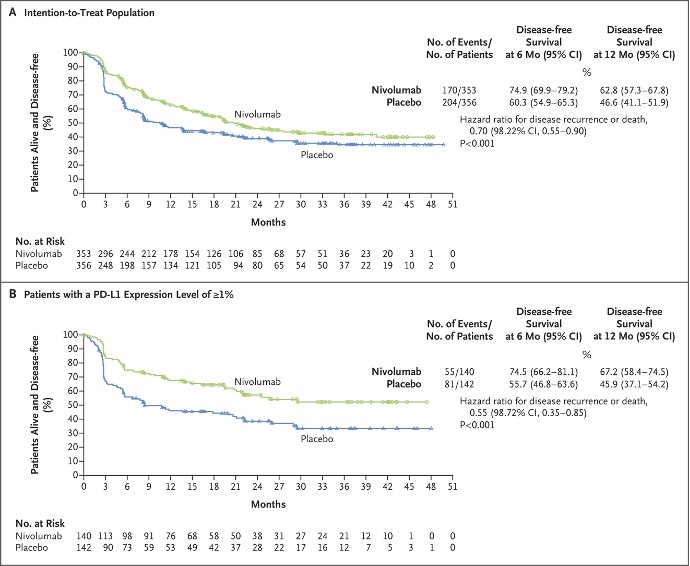
The third trial, IMvigor010, failed to demonstrate a significant disease-free survival benefit for adjuvant atezolizumab (19.4 versus 16.6 months; HR: 0.89, p=0.24) and no overall survival benefit was observed (18 months survival: 79% versus 73%).5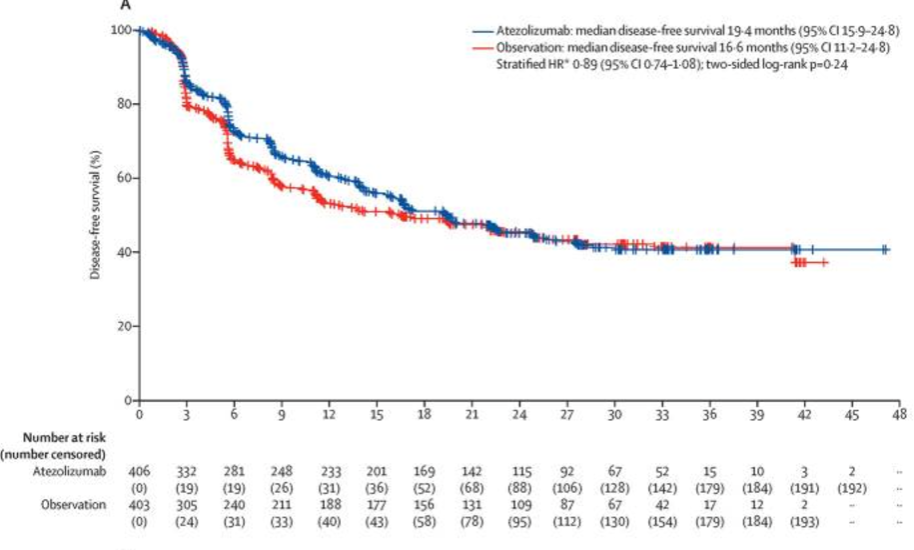
However, as Dr. Hoffman-Censits would detail later, the use of adjuvant ctDNA testing within this trial helped identify a cohort of patients (i.e., ctDNA+) that derived a survival benefit from adjuvant atezolizumab.6
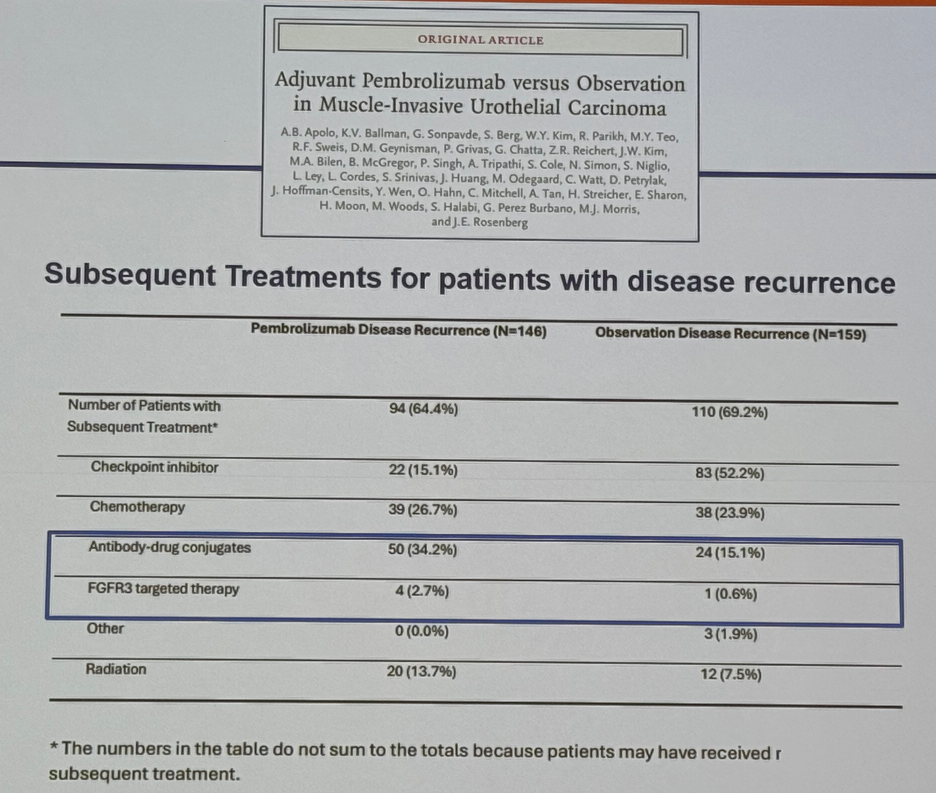
One important consideration for interpreting the results of these trials of adjuvant systemic therapy is to consider the subsequent treatments received at time of disease recurrence. In the AMBASSADOR trial of adjuvant pembrolizumab, 34% of patients in the pembrolizumab arm received antibody-drug conjugates (e.g., enfortumab vedotin), compared to only 15% of patients in the observation arm that had a disease recurrence. Similarly, more patients in the treatment arm received FGFR3-targeted therapy (2.7% versus 0.6%). Conversely, 52.2% of patients in the observation arm received a subsequent checkpoint inhibitor, compared to 15% in the intervention arm, which likely reflects the decision to utilize drugs with alternate mechanisms of action.
Another important outcome to consider in these patients is second progression-free survival (PFS2; i.e., the progression-free survival on the 2nd line therapy). In the CheckMate 274 trial, nivolumab-treated patients had superior median PFS outcomes (61.2 versus 47.1 months; HR: 0.79, 95% CI: 0.63–0.98), which indicates that the benefit of adjuvant nivolumab use is not limited to the early setting but is sustained with additional systemic therapy.
An emerging tool in the treatment paradigm of high-risk muscle invasive urothelial carcinoma patients in the adjuvant setting is ctDNA. Despite CheckMate 274 failing to demonstrate a survival benefit for adjuvant atezolizumab in the overall population, an ad hoc analysis of this trial clearly demonstrated that patients who were ctDNA+ derived a significant disease-free and overall survival benefit with adjuvant atezolizumab (median: 26 versus 16 months; HR: 0.59, 95% CI: 0.41–0.86).6
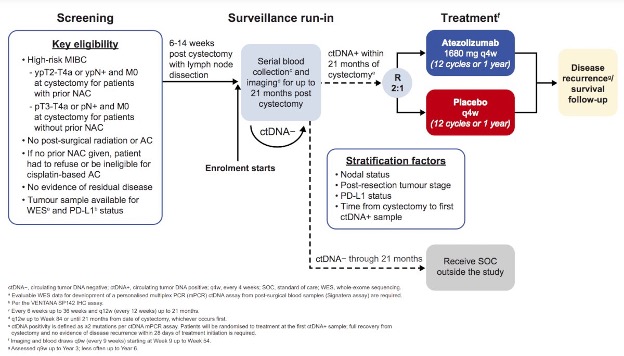
IMvigor011 is an ongoing, biomarker-driven trial that is randomizing high-risk muscle invasive bladder cancer patients post-radical cystectomy who have a ctDNA+ test within 21 months of surgery to either atezolizumab or placebo for one year.
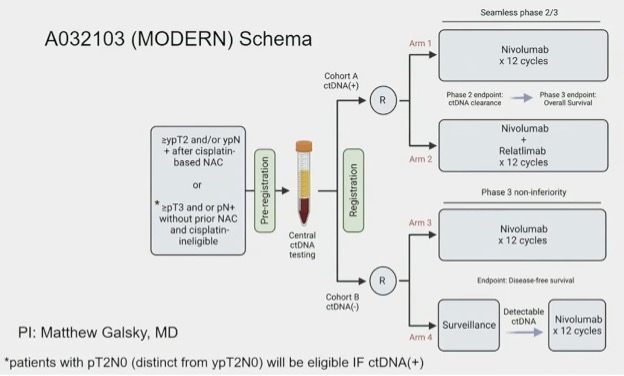
The MODERN (A032103) trial is a 4-arm trial, as illustrated below, that is randomizing high-risk, muscle invasive urothelial carcinoma patients as follows:
- ctDNA+: Nivolumab monotherapy versus nivolumab + relatlimab
- ctDNA-: Immediate nivolumab versus ‘salvage’ nivolumab at time of progression from ctDNA- to ctDNA+ status
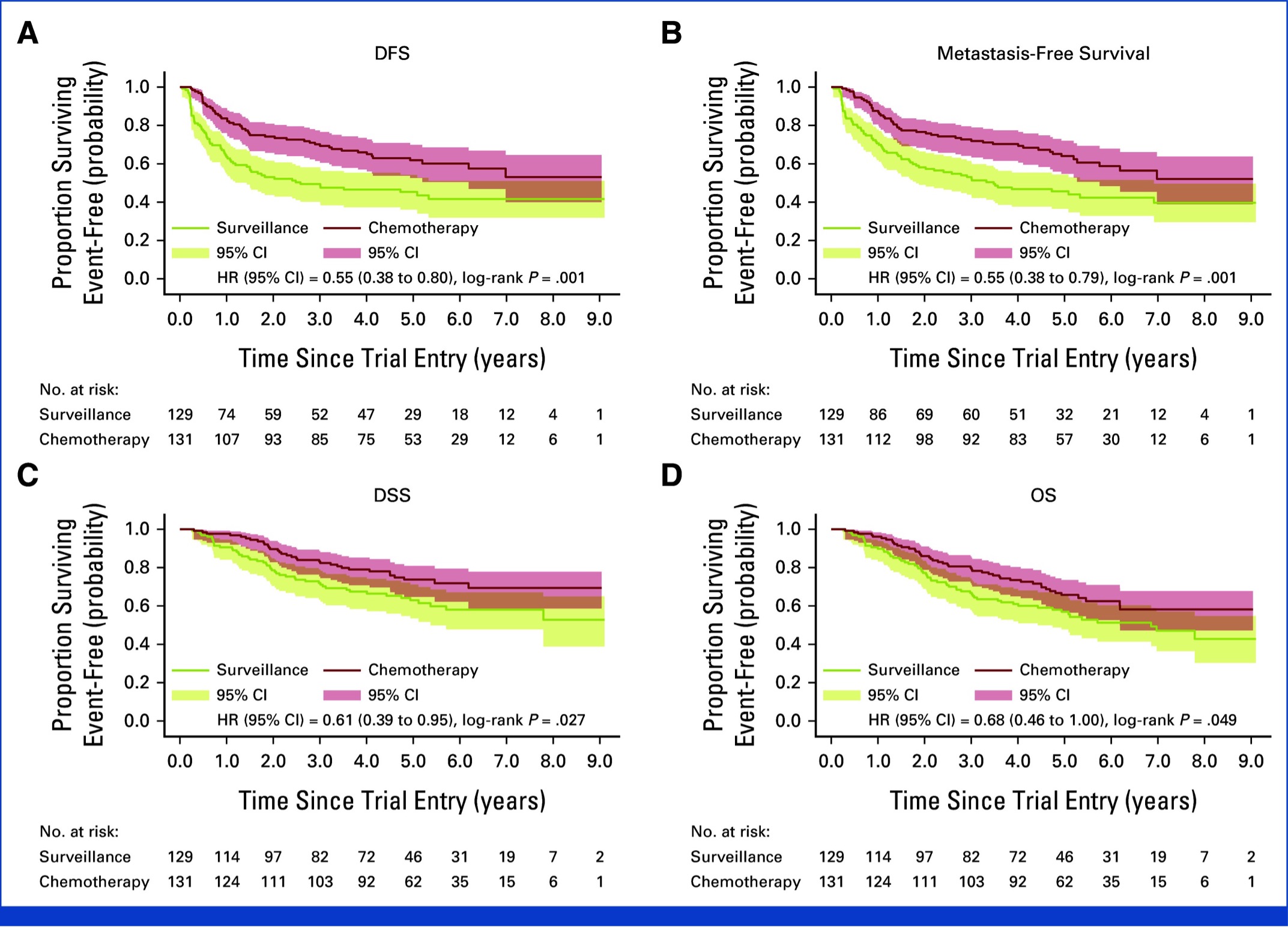
What about upper tract urothelial carcinoma? Platinum-based combination chemotherapy is approved for the adjuvant treatment of high-risk upper tract urothelial carcinoma patients following a nephroureterectomy, based on the results of the POUT trial.7,8 POUT was a phase III, open-label, randomised controlled trial at 71 hospitals in the UK that recruited upper tract urothelial carcinoma patients following nephroureterectomy staged as either pT2–T4 pN0–N3 M0 or pTany N1–3 M0 and were randomized to either surveillance or four 21-day cycles of platinum-based combination chemotherapy, administered within 90 days of surgery. The updated, long-term results of this trial were published this year in the Journal of Clinical Oncology and demonstrated a continued disease-free survival benefit for adjuvant chemotherapy (5-year: 62% versus 45%; HR: 0.55, p=0.001). The 5-year overall survival was 66% versus 57%, with a restricted mean survival benefit of 18 months with adjuvant chemotherapy.8 As a result, the latest AUA guidelines for upper tract urothelial carcinoma now support the use of adjuvant therapy in high-risk patients, if they had not received neoadjuvant chemotherapy.
While nivolumab is approved in the adjuvant setting for eligible high-risk upper tract urothelial carcinoma patients post-nephroureterectomy, Dr. Hoffman-Censits noted that subgroup analysis from CheckMate 274, as well as the other two trials in this space, has consistently demonstrated an absence of a survival benefit for adjuvant immune checkpoint inhibitors in patients with upper tract tumors. As such, it does not appear that patients with upper tract urothelial carcinoma derive the same benefit from adjuvant nivolumab as those with bladder cancer.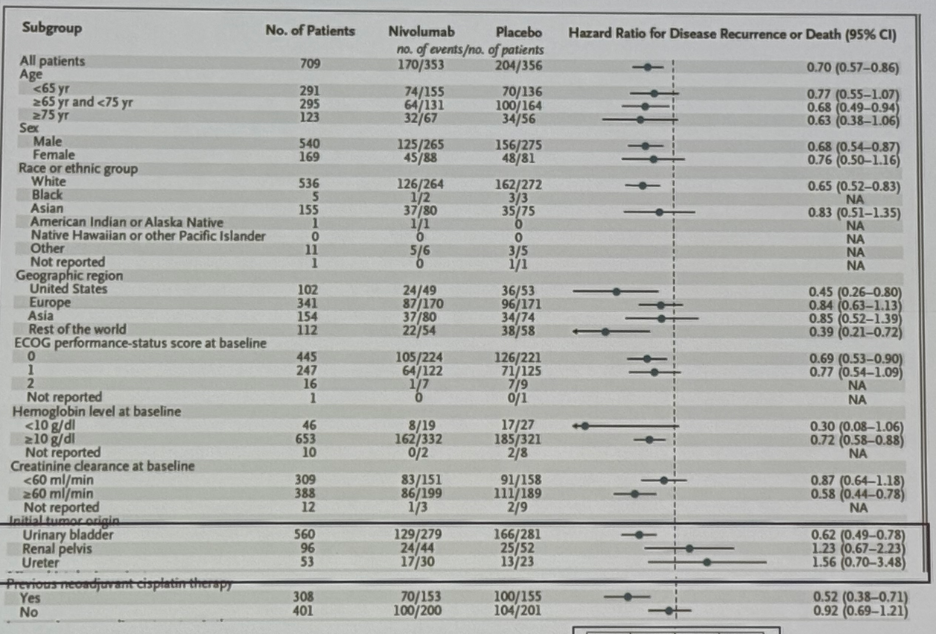
Conversely, subgroup analyses suggest that the survival benefit of nivolumab is most pronounced in patients with:
- Positive nodal status
- Bladder as primary site of disease
- Higher creatinine clearance at baseline
- Prior chemotherapy in the neoadjuvant setting
What about the use of immunotherapy in the perioperative setting (i.e., both neoadjuvant and adjuvant)? NIAGARA is a phase III, open label randomized trial that randomized cisplatin-eligible patients with muscle-invasive bladder cancer to receive neoadjuvant durvalumab plus gemcitabine–cisplatin every 3 weeks for four cycles, followed by radical cystectomy and adjuvant durvalumab every 4 weeks for eight cycles (durvalumab group), versus neoadjuvant gemcitabine–cisplatin followed by radical cystectomy alone (comparison group).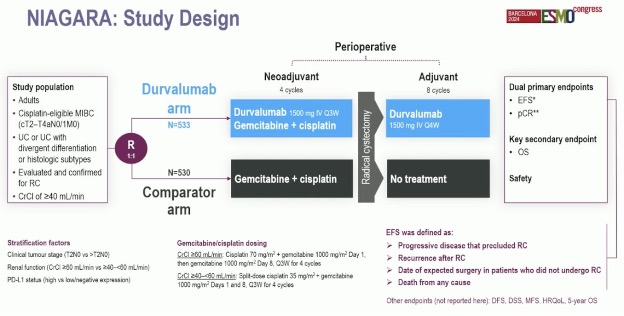
The use of perioperative durvalumab was associated with an improvement in 24-months event-free (68% versus 60%; HR: 0.68, p<0.001) and overall survivals (82% versus 75%, p=0.01).
There are currently numerous ongoing phase III studies of perioperative immune checkpoint inhibitors for muscle invasive bladder cancer, as summarized in the table below:

Dr. Hoffman-Censits concluded her presentation with the following take home messages:
- Adjuvant checkpoint inhibitors for high-risk urothelial cancer
- Improves disease-free survival, irrespective of PD-L1 status
- Overall survival benefit is pending
- The benefit persists in later line therapy
- Risks overtreatment with permanent checkpoint inhibitor toxicity
- Ongoing trials, such as MODERN, will evaluate the use of such agents at the time of ctDNA status conversion to minimize this risk
- Outcomes in rate populations (e.g., highest risk) remain underpowered and underexplored
- The trials do not capture screen failure for progressive disease
- Adjuvant therapy for high-risk upper tract disease:
- Adjuvant platinum should be administered for those who did not receive neoadjuvant chemotherapy
- Upper tract urothelial carcinoma patients were capped or enrolled at a≤20% in the three adjuvant studies of checkpoint inhibitors
- Subgroup analyses trends remain unexplained
- The intent-to-treat analyses for adjuvant nivolumab and pembrolizumab were positive for all comers
- Upper tract urothelial carcinoma is excluded from ongoing adjuvant and perioperative studies
Presented by: Jean Hoffman-Censits, MD, Associate Professor of Oncology, Department of Medicine, Johns Hopkins University, Baltimore, MD
Written by: Rashid K. Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX between December 3rd and 6th, 2024
References:
- FDA approves nivolumab for adjuvant treatment of urothelial carcinoma. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nivolumab-adjuvant-treatment-urothelial-carcinoma. Accessed on December 4, 2024.
- Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021; 384(22):2102-14.
- Galsky MD, Witjes JA, Gschwend JE, et al. Adjuvant Nivolumab in High-Risk Muscle-Invasive Urothelial Carcinoma: Expanded Efficacy From CheckMate 274. J Clin Oncol. 2024; JCO2400340.
- Apolo AB, Ballman KV, Sonpavde G, et al. Adjuvant Pembrolizumab versus Observation in Muscle-Invasive Urothelial Carcinoma. N Engl J Med. 2024.
- Bellmunt J, Hussain M, Gschwend JE, et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): A multicentre, open-label, randomized, phase 3 trial. Lancet Oncol. 2021; 22(4):525-37.
- Powles T, Assaf ZJ, Davarpanah N, et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature. 2021; 595(7867):432-7.
- Birtle A, Johnson M, Chester J, et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): A phase 3, open-label, randomized controlled trial. Lancet 2020; 395(10232):1268-77.
- Birtle AJ, Jones R, Chester J, et al. Improved Disease-Free Survival With Adjuvant Chemotherapy After Nephroureterectomy for Upper Tract Urothelial Cancer: Final Results of the POUT Trial. J Clin Oncol. 2024; 42(13):1466-71.
- Powles T, Catto JWF, Galsky MD, et al. Perioperative Durvalumab with Neoadjuvant Chemotherapy in Operable Bladder Cancer. N Engl J Med. 2024; 391:1773-86.


