(UroToday.com) The 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas was host to the Abstract/Posters Session. Dr Joaquim Bellmunt presented the disease-free survival and overall survival in patients with high-risk muscle-invasive bladder cancer who have persistent circulating tumor DNA-negative biomarker status post-cystectomy in a surveillance analysis of the IMvigor011 study.
There is data suggesting that circulating tumor DNA (ctDNA) can help identify patients with muscle-invasive bladder cancer (MIBC) who are at risk of relapse after radical cystectomy.1,2 The Phase III IMvigor010 study (NCT02450331) investigated the efficacy and safety of atezolizumab as an adjuvant therapy for patients with muscle-invasive urothelial carcinoma who are at high risk of recurrence following radical cystectomy or nephroureterectomy with lymph node dissection. Eligible patients had ypT2-4a or ypN+ tumors following neoadjuvant chemotherapy, or pT3-4a or pN+ tumors if they did not receive neoadjuvant chemotherapy.
This study performed landmark ctDNA testing in a high-risk MIBC population. The investigators found that approximately 30% of ctDNA− patients still experienced a disease-free survival (DFS) event, as shown in the Kaplan-Meier graph below.3
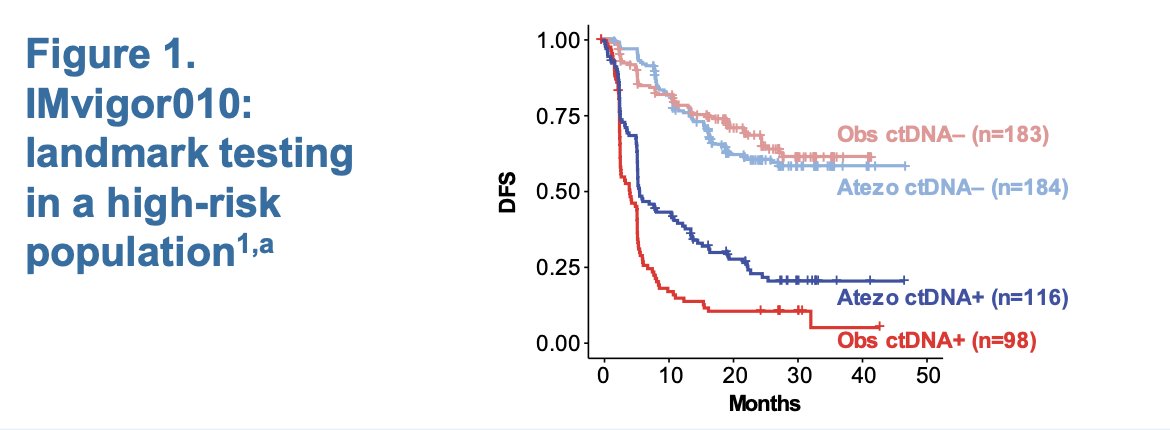
IMvigor011 is a global, double-blind, randomized Phase III study assessing the efficacy of atezolizumab versus placebo in patients with high-risk MIBC who had ctDNA+ status within 12 months post-radical cystectomy
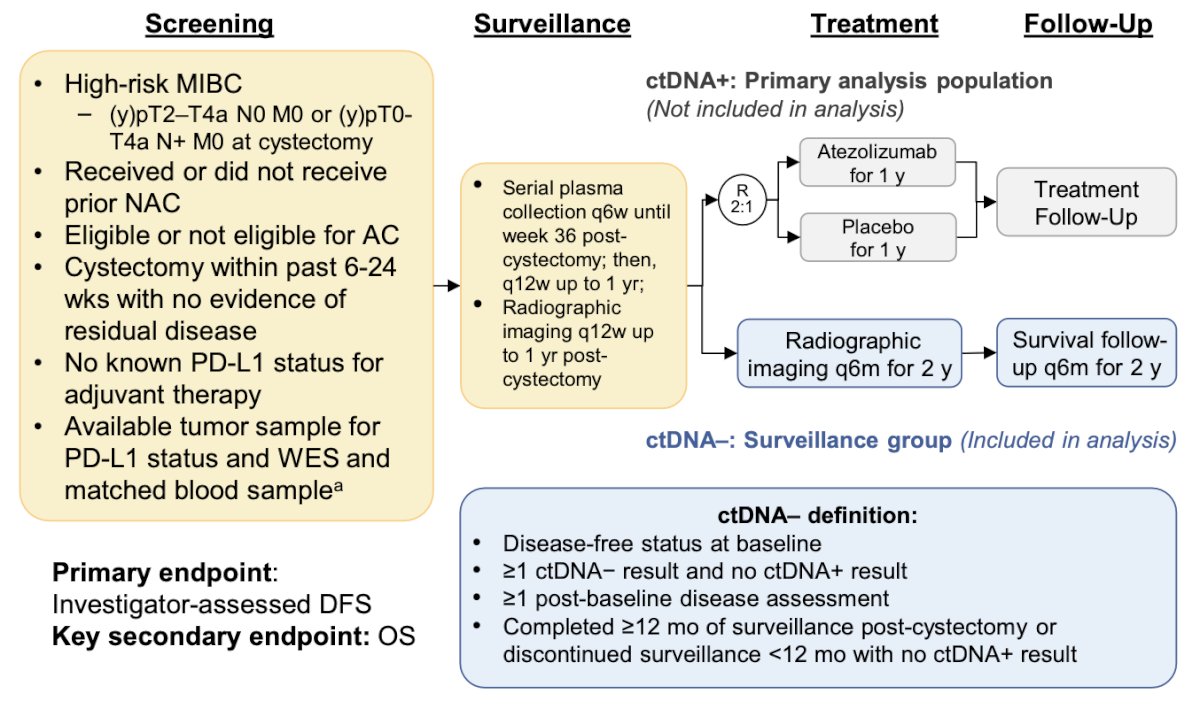
IMvigor011 (NCT04660344) is a global, double-blind, randomized Phase III study assessing the efficacy of atezolizumab versus placebo in patients with high-risk MIBC who are ctDNA positive post-cystectomy.4 The surveillance cohort analysis of the IMvigor011 study examined clinical outcomes in patients who maintained a ctDNA− status for 12 months post-cystectomy. The study design is illustrated in the figure below:
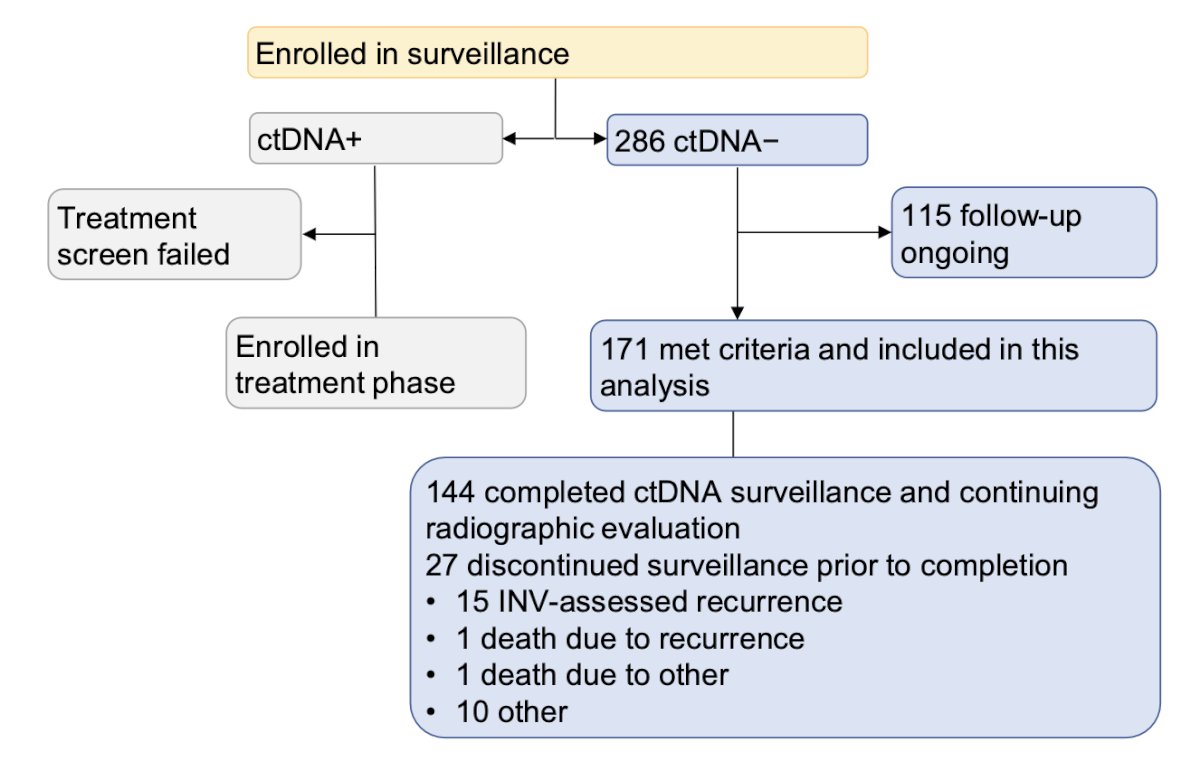
A total of 286 patients were identified with ctDNA-status. Of these, 171 met the inclusion criteria and were incorporated into the surveillance analysis. Finally, 144 patients completed ctDNA surveillance and continued with radiographic evaluation.
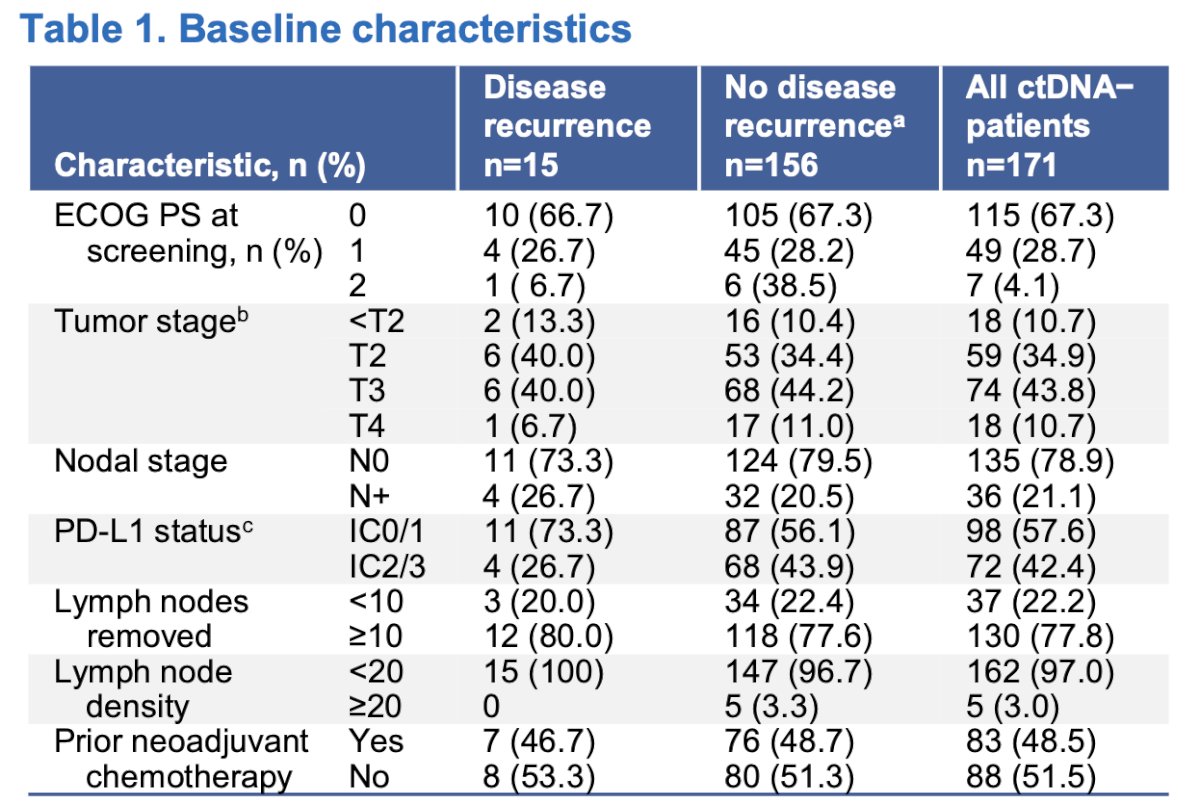
In the overall ctDNA− sub-cohort, the median age was 69 years. The majority of patients were male (78.9%) and White (56.1%), with an ECOG-PS of 0 in 67.3% of the cases. Within the surveillance cohort, 8.8% experienced disease recurrence. Recurrence occurred at distant sites in 11 patients and at local sites in 4 patients.
The 12 and 18 month DFS rates were 92% and 88%, respectively in the surveillance cohort (ctDNA-) as illustrated in the Kaplan-Meier graphic below:
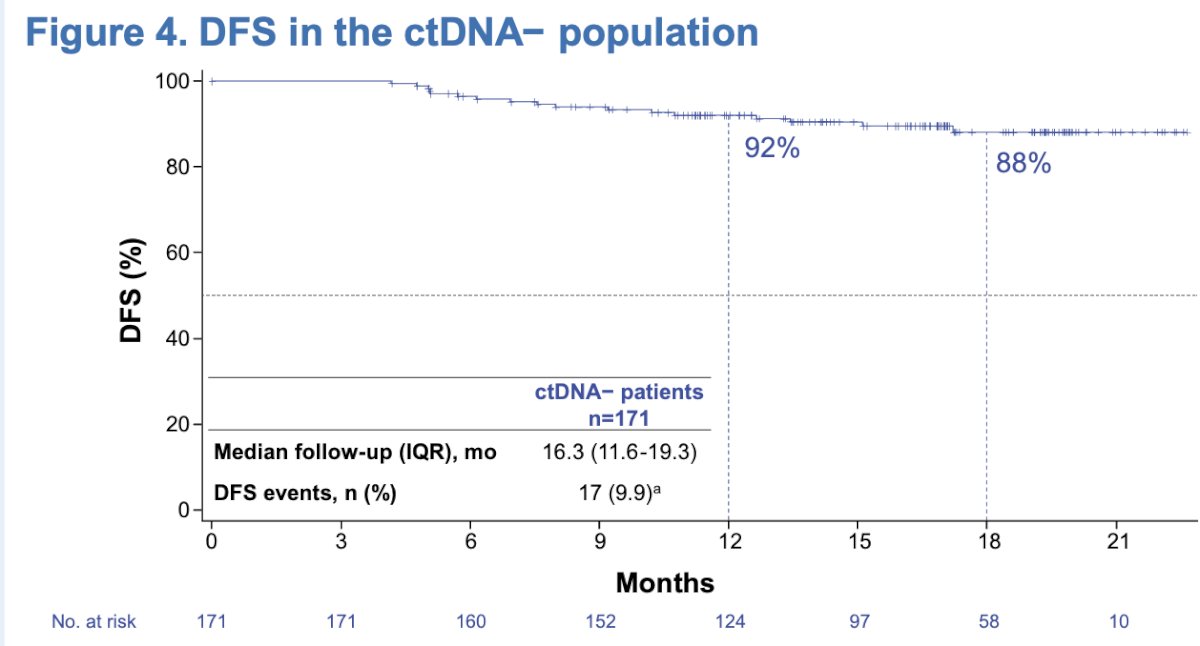
In an exploratory analysis of DFS outcomes according to PD-L1 subgroups (IC0/1 vs. IC2/3), the median DFS was not-reached in both subgroups and DFS outcomes were similar regardless of PD-L1 status.
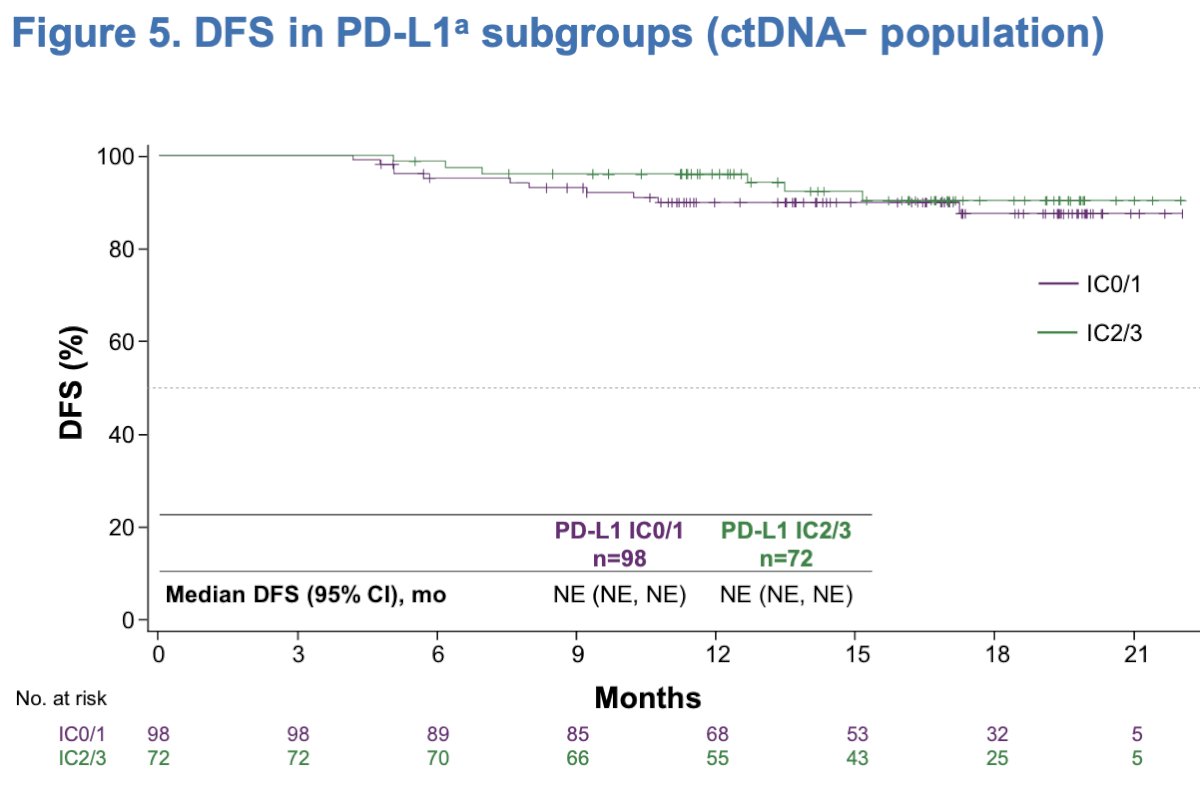
The overall survival (OS) analysis is still immature, the 12 and 18-month OS rates were 100% and 98%, respectively, with a median follow-up of 16.3 months and only OS events, registered.
Dr Bellmunt concluded his poster presentation with the following messages:
- This exploratory analysis from the IMvigor011 surveillance cohort suggested that serial ctDNA testing may have greater clinical utility than landmark ctDNA testing as a risk stratification tool for patients with high-risk MIBC.
- Within the ctDNA− sub-cohort, ctDNA status aids in selecting patients for surveillance with a favorable clinical prognosis regardless of PD-L1 status.
- These data increase confidence that patients with persistent ctDNA− status post-cystectomy may be spared adjuvant treatment.
Presented by: Joaquim Bellmunt, MD, PhD, Associate Professor at Harvard Medical School and Director of Bladder Cancer Center at Genitourinary Oncology Program of Dana-Farber Cancer Institute, Boston, MA.
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 Society of Urologic Oncology (SUO) Annual Meeting, Dallas, TX, Tues, Dec 3 – Fri, Dec 6, 2024.
References:- Powles TB, et al. ESMO IO 2020. Abs 1O.
- Christensen E, Birkenkamp-Demtröder K, Sethi H, Shchegrova S, Salari R, Nordentoft I, Wu HT, Knudsen M, Lamy P, Lindskrog SV, Taber A, Balcioglu M, Vang S, Assaf Z, Sharma S, Tin AS, Srinivasan R, Hafez D, Reinert T, Navarro S, Olson A, Ram R, Dashner S, Rabinowitz M, Billings P, Sigurjonsson S, Andersen CL, Swenerton R, Aleshin A, Zimmermann B, Agerbæk M, Lin CJ, Jensen JB, Dyrskjøt L. Early Detection of Metastatic Relapse and Monitoring of Therapeutic Efficacy by Ultra-Deep Sequencing of Plasma Cell-Free DNA in Patients With Urothelial Bladder Carcinoma. J Clin Oncol. 2019 Jun 20;37(18):1547-1557. doi: 10.1200/JCO.18.02052. Epub 2019 May 6. PMID: 31059311.
- Bellmunt J, Hussain M, Gschwend JE, Albers P, Oudard S, Castellano D, Daneshmand S, Nishiyama H, Majchrowicz M, Degaonkar V, Shi Y, Mariathasan S, Grivas P, Drakaki A, O'Donnell PH, Rosenberg JE, Geynisman DM, Petrylak DP, Hoffman-Censits J, Bedke J, Kalebasty AR, Zakharia Y, van der Heijden MS, Sternberg CN, Davarpanah NN, Powles T; IMvigor010 Study Group. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021 Apr;22(4):525-537. doi: 10.1016/S1470-2045(21)00004-8. Epub 2021 Mar 12. PMID: 33721560; PMCID: PMC8495594.
- Jackson-Spence F, Toms C, O'Mahony LF, Choy J, Flanders L, Szabados B, Powles T. IMvigor011: a study of adjuvant atezolizumab in patients with high-risk MIBC who are ctDNA+ post-surgery. Future Oncol. 2023 Mar;19(7):509-515. doi: 10.2217/fon-2022-0868. Epub 2023 Apr 21. PMID: 37082935.


