(UroToday.com) The 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX between December 3rd – 6th, 2024 was host to a bladder cancer session. Dr. Mark Tyson provided a comprehensive overview of the emerging intravesical therapy options for the treatment of non-muscle invasive bladder cancer (NMIBC), discussing their mechanisms of action, efficacy, tolerability, as well as highlighting important financial and access considerations.
Dr. Tyson noted that there are two potential ways to organize how this topic can be approached. First, it can be approached by disease state, as summarized in the left column below, and this is the approach/paradigm most clinicians are familiar with. However, we could also frame this discussion around treatment modalities, focusing on the tools and innovations available to us. And while this approach is less systematic, Dr.Tyson argued that organizing by treatment modalities allows us to spotlight innovations and compare different technologies across disease states. In this talk, Dr. Tyson adopted a hybrid approach where he adopted the treatment modality framework within a disease state or two to ensure that this rapidly evolving clinical and therapeutic landscape is comprehensively addressed.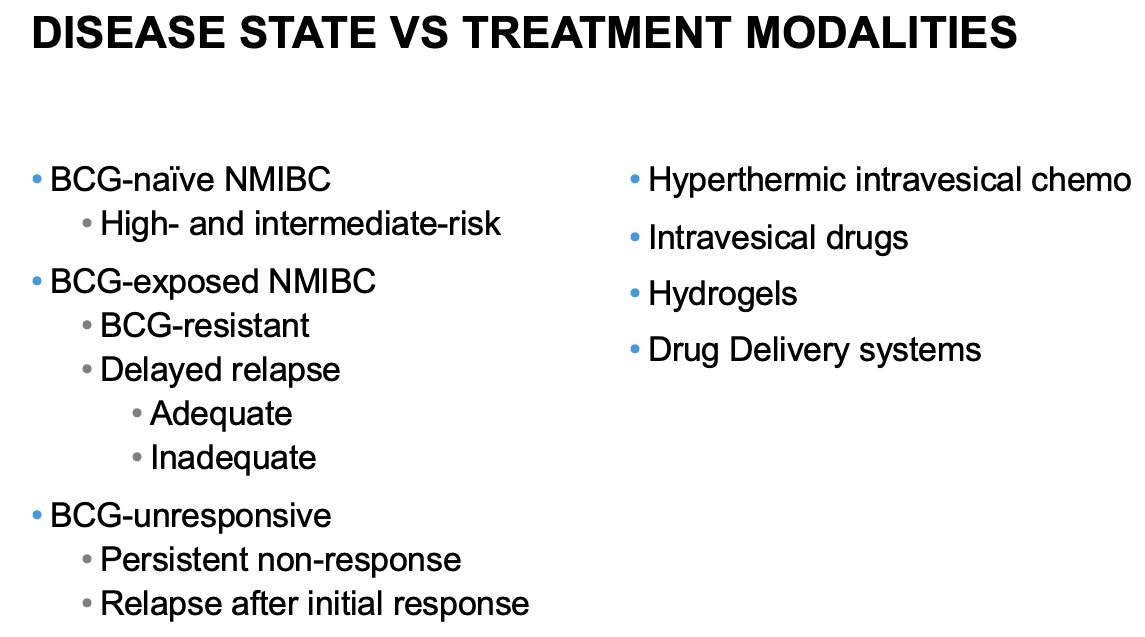
First, Dr. Tyson addressed the use of intravesical drugs and drug delivery systems for patients with BCG unresponsive disease. Summarized below is the timeline of FDA drug approvals for the treatment of NMIBC, with those highlighted by the red box representing drugs approved for patients with BCG-unresponsive disease: valrubicin (1998), pembrolizumab (2020), nadofaragene firadenovec (2022), and N803 (2024).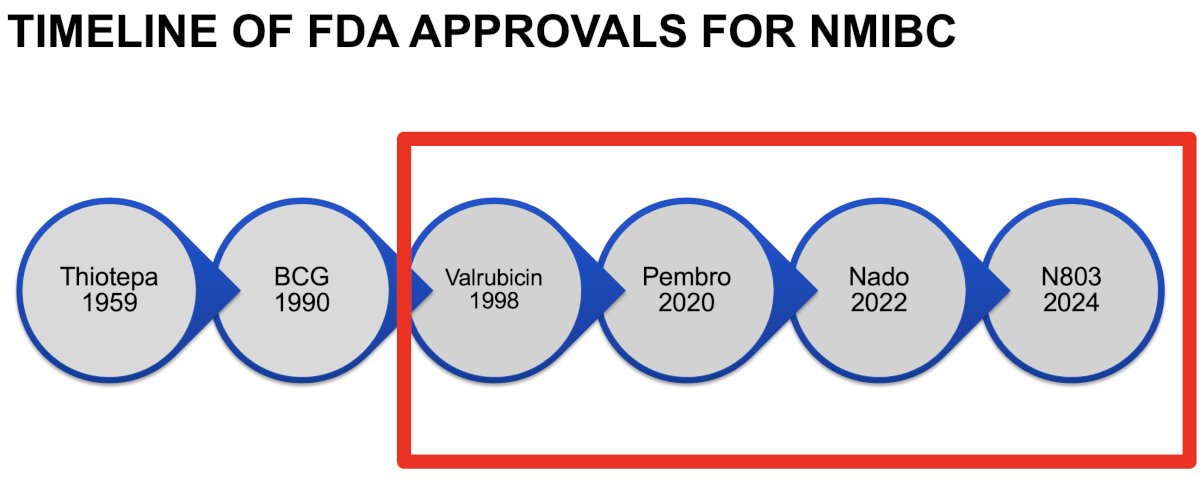
Summarized in the table below are the key trials of intravesical drug therapy +/- systemic therapy for the treatment of BCG unresponsive NMIBC. What is striking from this table is the diverse nature of these drugs, with varying mechanisms of action. Cretostimogene is an oncolytic immunotherapy, which is being evaluated in BOND-003 and CORE-001 (with concurrent pembrolizumab)1. EG-70 is an RIG-1 agonist + IL-12 agent (LEGEND trial).2 TAR-200 is a promising, novel intravesical drug delivery system that allows for the sustained release of gemcitabine and is being evaluated in BCG-unresponsive CIS patients. The combination of N-803 (IL-15) + BCG was evaluated in the QUILT 3.032 trial.3 Nadofaragene firadenovec relies on a transfected adenovirus delivery system that leads to IFN release.4 Pembrolizumab has been evaluated in the KEYNOTE-057 trial and is a systemic immune checkpoint inhibitor.5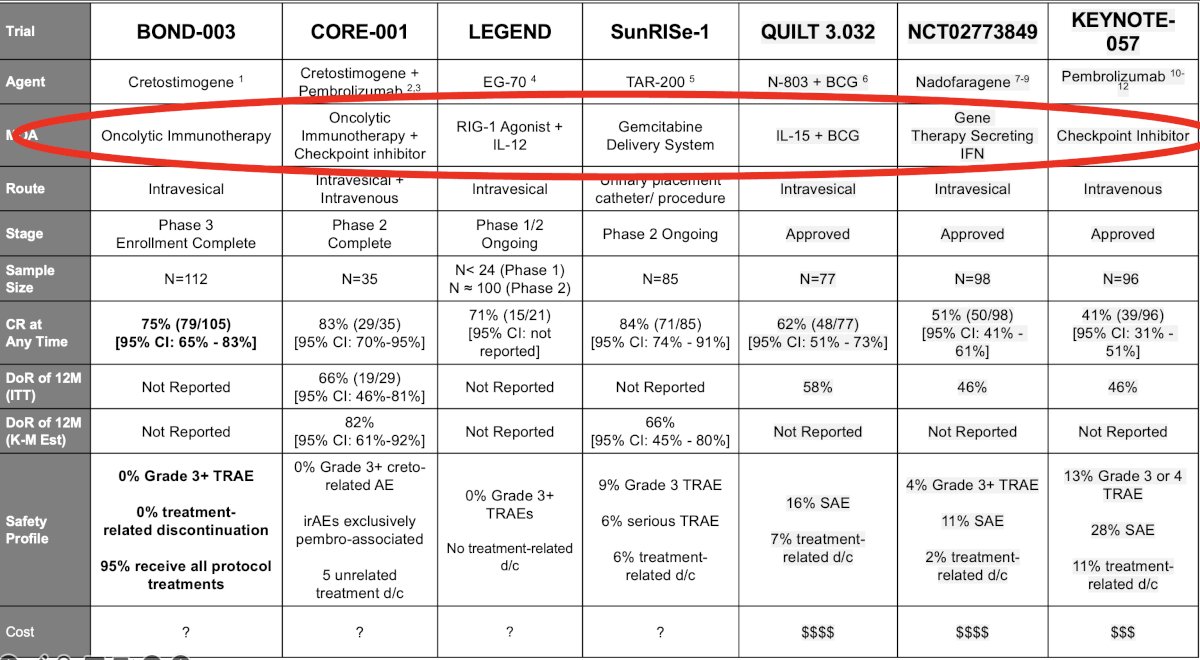
The complete response at any time for these drugs is impressive with cretostimogene (+/- pembrolizumab) and TAR-200 all achieving complete responses ≥75%. However, Dr. Tyson emphasized that the duration of a complete response, and not only achieving a complete response, is of utmost importance when comparing these agents. As illustrated below, we see a sizeable difference between the complete response at any time and 1 year for these agents: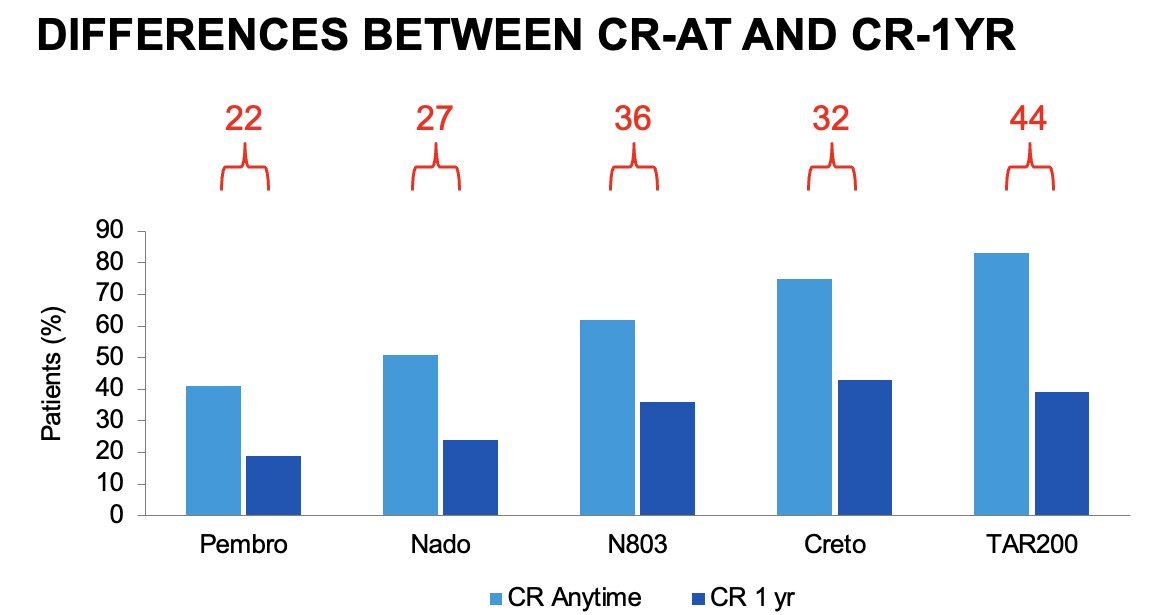
The duration of response is the ‘great equalizer’, and, as summarized below, we see that the duration of response for these agents at 1 year, where available, ranges between 46% and 66%.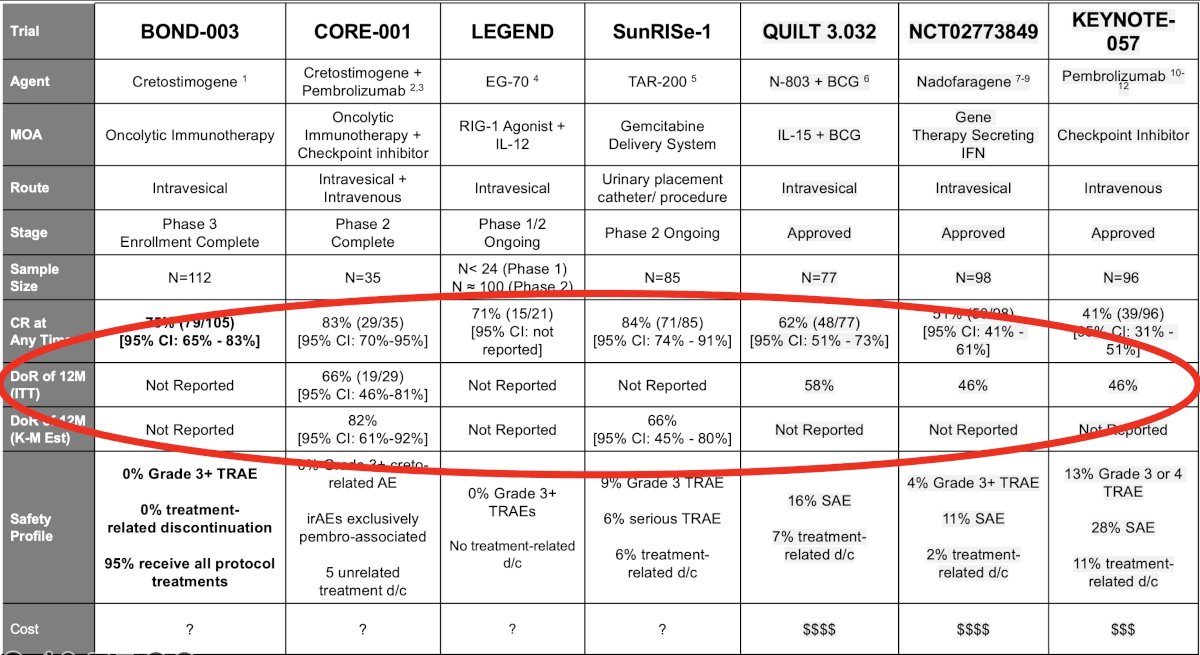
Direct comparisons of the efficacy and safety of these agents is limited by inherent differences in the patient populations, treatment schedules, and surveillance regimens, including the requirement for bladder biopsies at set time intervals. As such, Dr. Tyson noted that ‘we have no idea how these drugs compare’. We can, however, make some crude comparisons between these agents. If we consider the complete response rate at 1 year, we see significant differences between cretostimogene + pembrolizumab (~60%) and pembrolizumab monotherapy (~20%). As such, we can deduce that cretostimogene + pembrolizumab probably outperforms pembrolizumab monotherapy in this setting. Conversely, there are minimal differences in the 1-year complete response rates between cretostimogene monotherapy, TAR-200, and N-803, making any efficacy comparisons ‘debatable’.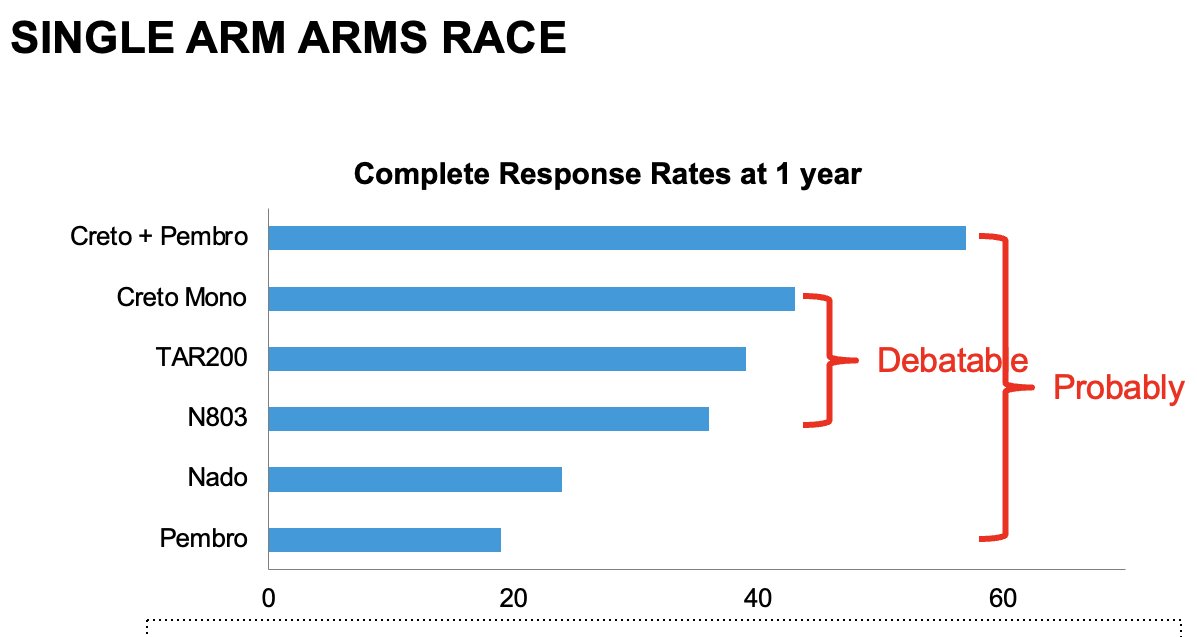
Other important considerations are the drugs’ safety profile, with these drugs overall having favorable severe adverse events profiles, and the associated costs of these agents.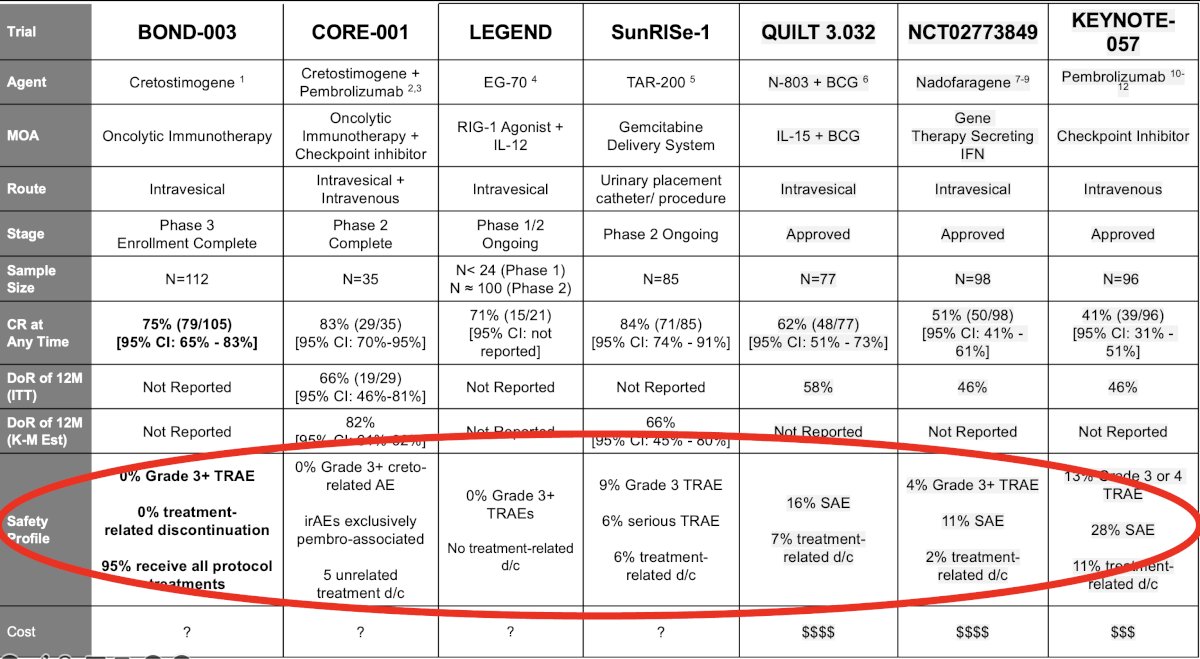
To date, intravesical gemcitabine + docetaxel remains the de facto standard of care in the BCG unresponsive NMIBC setting. It is not currently FDA approved, due to the absence of level 1 (i.e., randomized trials) evidence supporting its use in this setting; however, this combination has demonstrated excellent and durable efficacy outcomes in large single center series.6 Furthermore, this combination is cheap, familiar to prescribing physicians and nurses, and has no significant side effects.
|
Therapy |
Average Retail Price (USD) |
|
Gemcitabine1 |
$26 per 1, 26.3ML of 1GM/26.3ML |
|
Docetaxel2 |
$158 per 2, 1 ML 20MG/ML, vials |
|
Pembrolizumab3 |
$22,674 per dose |
|
Nadofaragene4 |
$60,000 per dose |
|
Nogapendekin5 |
$35,800 per dose |
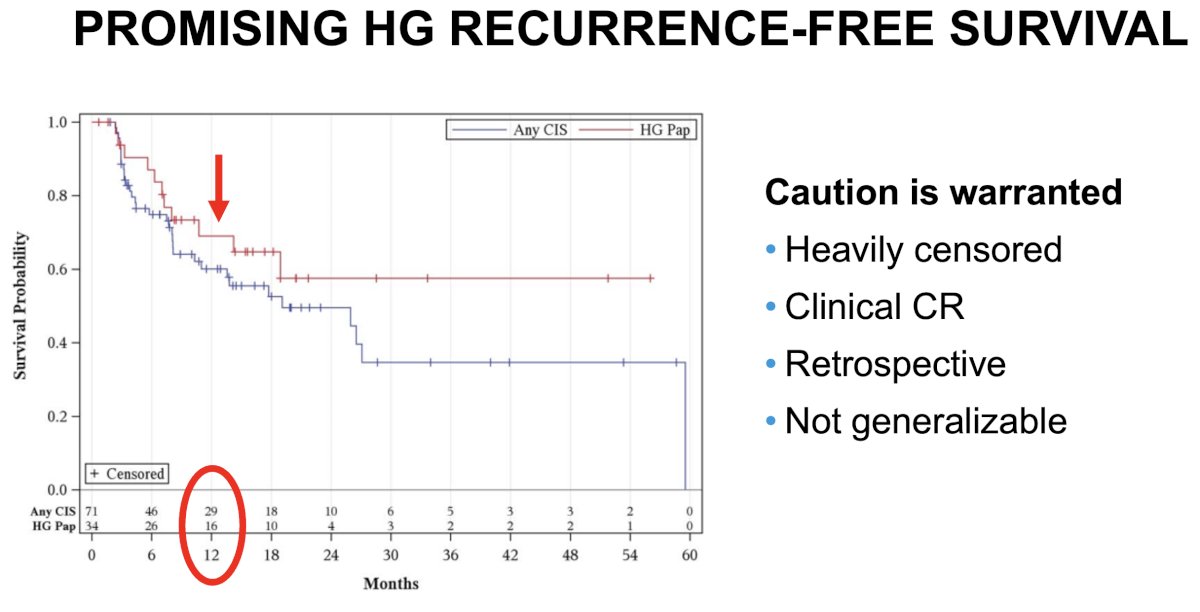
While the study by Steinberg et al. in 2020 demonstrated excellent efficacy outcomes for gemcitabine + docetaxel in BCG-unresponsive NMIBC patients,6 Dr. Tyson advised that caution is warranted when interpreting the results of this study. Important limitations of clinical relevance in this study include:
- The high degree of early censoring
- Reliance on a clinical complete response, as opposed to scheduled biopsy regimens
- Retrospective nature of analysis
- Lack of generalizability
To date, radical cystectomy remains the gold standard treatment for BCG unresponsive NMIBC, and this recommendation is supported by numerous guidelines: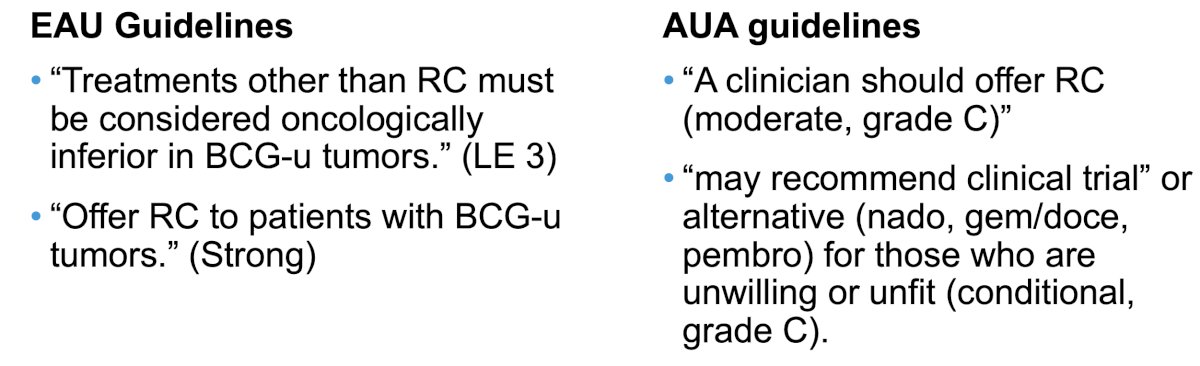
Recently, Nishimura et al. published the results of a series of 141 BCG-unresponsive NMIBC patients who underwent a radical cystectomy. The final pathology was as follows:
- pT0, pTa, or pTis: 43%
- pT1: 23%
- ≥pT2: 34%
Overall, survival outcomes were excellent for these patients, with overall survival worsening with more advanced pathologic stages.7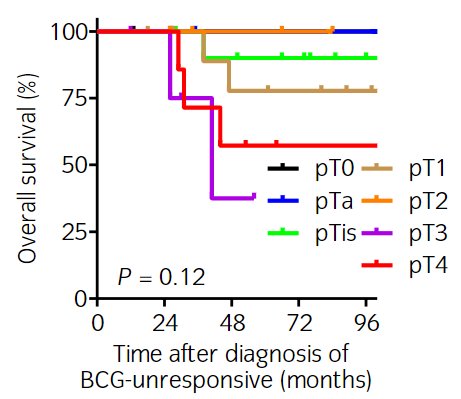
What is Dr. Tyson’s ‘final verdict’ for radical cystectomy:
- It is a reasonable option in all patients, especially those with:
- High grade T1, high volume Ta/Tis
- Variant histology
- Involvement of the urethra and upper tracts
- However enrolment in a clinical trial or other intravesical alternative is reasonable too, particularly for disease that is resectable.
Presented by: Mark Tyson, MD, MPH, Associate Professor of Urology, Mayo Clinic Arizona, Scottsdale, AZ
Written by: Rashid K. Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX between December 3rd and 6th, 2024
References:- Li R, Shah PH, Stewart TF, et al. Oncolytic adenoviral therapy plus pembrolizumab in BCG-unresponsive non-muscle-invasive bladder cancer: the phase 2 CORE-001 trial. Nat Med. 2024; 30:2216-23.
- Bryce R, Tosone C, Sullivan JC, et al. A phase 1/2 study of EG-70 (detalimogene voraplasmid) intravesical monotherapy for patients with BCG-unresponsive non-muscle invasive bladder cancer with carcinoma in situ. J Clin Oncol. 2024; 42:Number 16_Suppl.
- Chamie K, Chang SS, Kramolowsky E, et al. IL-15 Superagonist NAI in BCG-Unresponsive Non–Muscle-Invasive Bladder Cancer. NEJM Evidence. 2022; 2(1).
- Boorjian SA, Alemozaffar M, Konety BR, et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: A single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. 2020: S1470-2045(20)30540-4.
- Balar AV, Kamat AM, Kulkarni GS, et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): An open-label, single-arm, multicenter, phase 2 study. Lancet Oncol. 2021; 22(7):919-30.
- Steinberg RL, Thomas LJ, Brooks N, et al. Multi-Institution Evaluation of Sequential Gemcitabine and Docetaxel as Rescue Therapy for Nonmuscle Invasive Bladder Cancer. J Urol. 2020; 203(5):902-9.
- Nishimura N, Miyake M, Iida K, et al. Prognostication in Japanese patients with bacillus Calmette-Guérin-unresponsive non-muscle-invasive bladder cancer undergoing early radical cystectomy. Int J Urol. 2022; 29(3):242-9.


