(UroToday.com) The 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX between December 3rd – 6th, 2024 was host to a bladder cancer session. Dr. Joshua Meeks discussed future trial designs for drugs in the BCG-unresponsive non-muscle invasive bladder cancer (NMIBC) space, arguing that it may be time to move past the ‘historic’ single arm registration trials.
Dr. Meeks noted that one of the biggest problems with BCG unresponsive CIS in 2024 is that all approved agents were based on the results of single arm trials, and there have been no comparisons of safety or efficacy. While single arm trials have been successful for facilitating new drug approvals, they have their limitations. Another important issue with these trials is the heterogeneous nature of the patient populations, who have a wide range of BCG exposure histories. Some have received full BCG doses, whereas others have received 1/3 doses. The number of BCG induction and maintenance cycles administered varies, and the time from last BCG instillation to recurrence is also variable. There may also be differences in the cancer volume present at the time of drug therapy. Some patients may have negative white light cystoscopies, but have blue light-positive lesions, as illustrated below. Should all patients be ‘screened’ into these trials with blue light cystoscopies?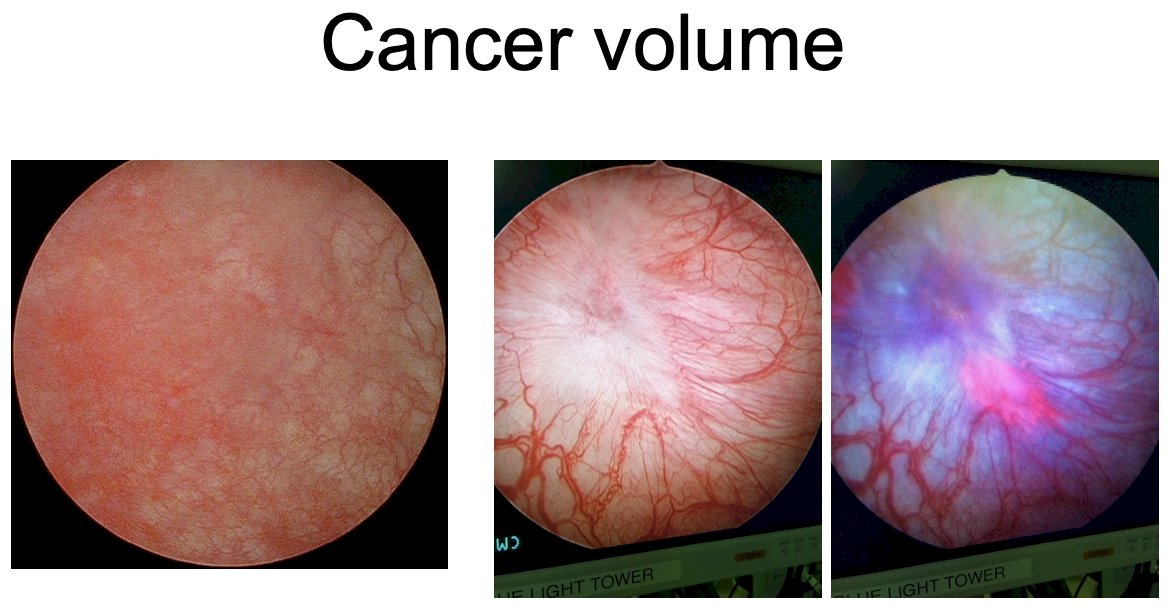
Should we incorporate urinary biomarkers, such as utDNA, into the management paradigm to help facilitate early detection of more aggressive variants that require treatment intensification versus those who have absent disease and may be candidates for treatment de-intensification? Presented at ASCO GU 2024, St-Laurent et al. demonstrated that urinary comprehensive genomic profile (uCGP) at baseline and after 4 cycles of atezolizumab treatment can identify genomic patterns associated with an increased risk of high-grade disease persistence, recurrence, or progression in BCG-unresponsive NMIBC treated with immune checkpoint inhibition.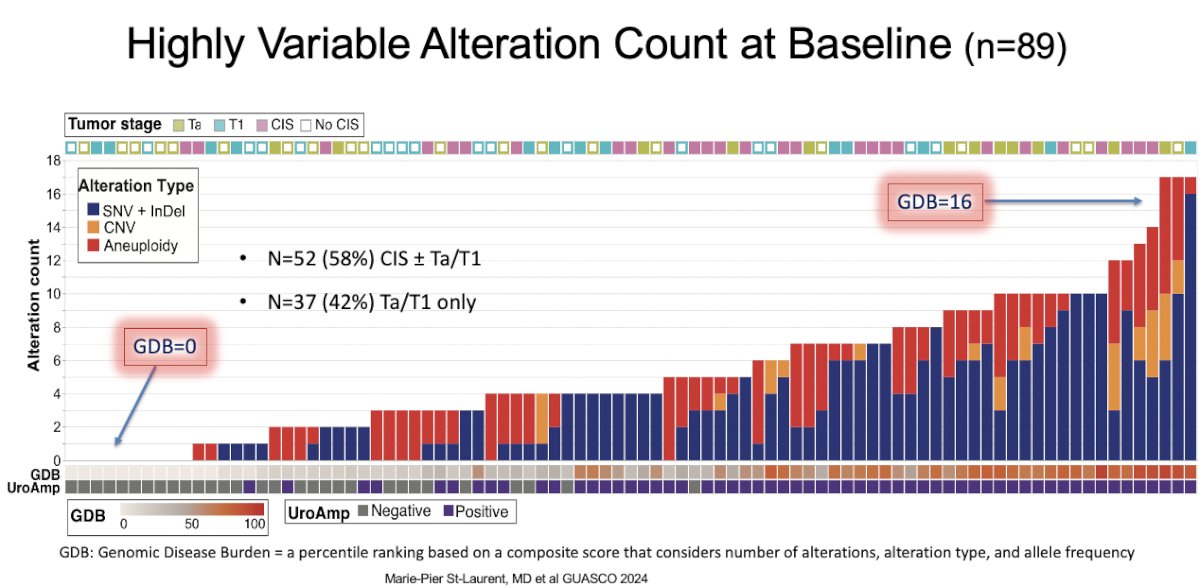
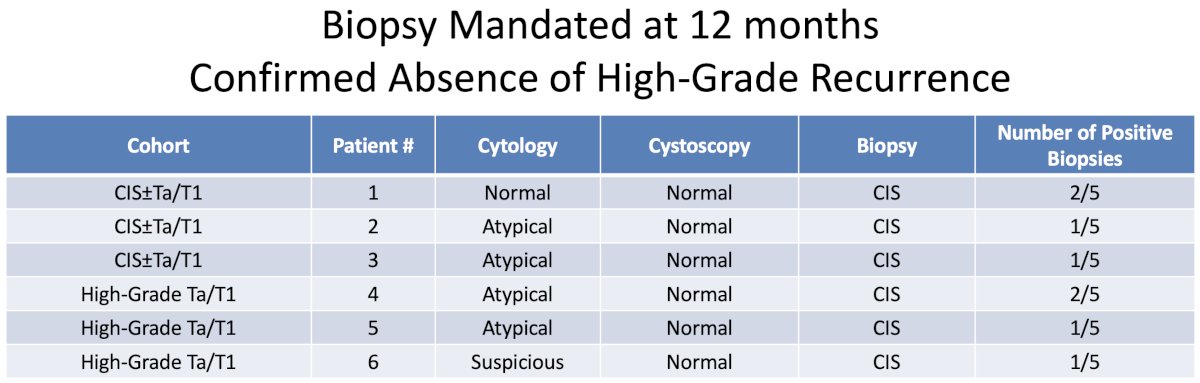
Another important consideration when interpreting the results of these trials of BCG-unresponsive NMIBC is the requirement (or lack thereof) for mandated bladder biopsies at set time intervals? In the nadofaragene trial, 6 patients with a normal cystoscopy were found to have CIS at the time of the protocol-mandated biopsy at 12 months, of whom 5 would have been considered free from high-grade recurrence without the mandatory biopsy.1 Based on clinical features alone, the response rate at 12 months would have been reported as:
- CIS ± Ta/T1 cohort: 27% complete response
- High grade Ta/T1 cohort: 48% high grade recurrence-free survival
How did we get to single-arm trials designs as a path to FDA approval? In 2013, the U.S. Food and Drug Administration (FDA) and the American Urological Association (AUA) co-sponsored a public workshop held in San Diego, CA to review potential trial designs for the development of new therapies for NMIBC.2
The following guidance statements were proposed:
- Single-arm trials are appropriate where randomized trial is unethical or not feasible
- Randomizing BCG-unresponsive patients to placebo or minimally effective drug raises ethical concerns.
- Single-arm trials are appropriate because “currently, no effective medical therapies are available, and the only alternative is radical cystectomy.”
- If effective therapies become available, “a randomized trial may be appropriate.”
Prospective benchmarks for potential agent approvals in this space were set as follows:
- 6 months complete response rate: 40–50%
- 18–24 months durable response: ≥30%, with the 95% confidence interval excluding 20%
Conversely, the International Bladder Cancer Group set the following benchmarks: for BCG-unresponsive CIS:3
- Complete response rate of 50% at 6 months
- Durable response rates of 30% at 12 months and 25% at 18 months
But what about a BCG re-challenge in patients with ‘BCG-unresponsive NMIBC’? Results from the MD Anderson experience published earlier this year suggests that, overall, 75% of patients remain disease free, with a 69% durable response at 12 months.4 This raises the question as to whether we should re-think the role of a BCG re-challenge, and whether this would be an appropriate control arm in randomized trials of drugs in this space? The caveat here is the ongoing BCG shortage worldwide that limits BCG administration, even in BCG-naïve patients.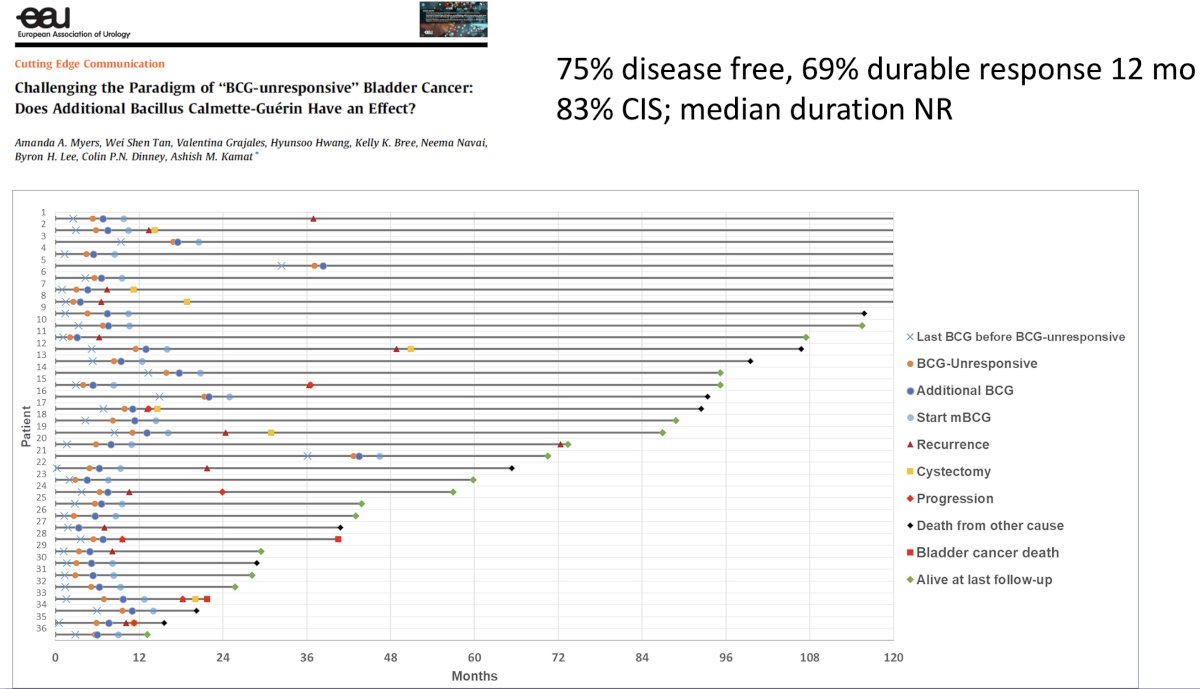
What is our vision of therapy for BCG unresponsive CIS in 2024 and beyond? Dr. Meeks argued that there should be three stakeholders involved in this disease space:
- Patient: Who wish to remain disease-free, retain their native bladder, and remain free of harm
- Providers: Who want to know where to start, where to move next, and and when to throw in the towel
- System: Determine options for different patients, sustainability
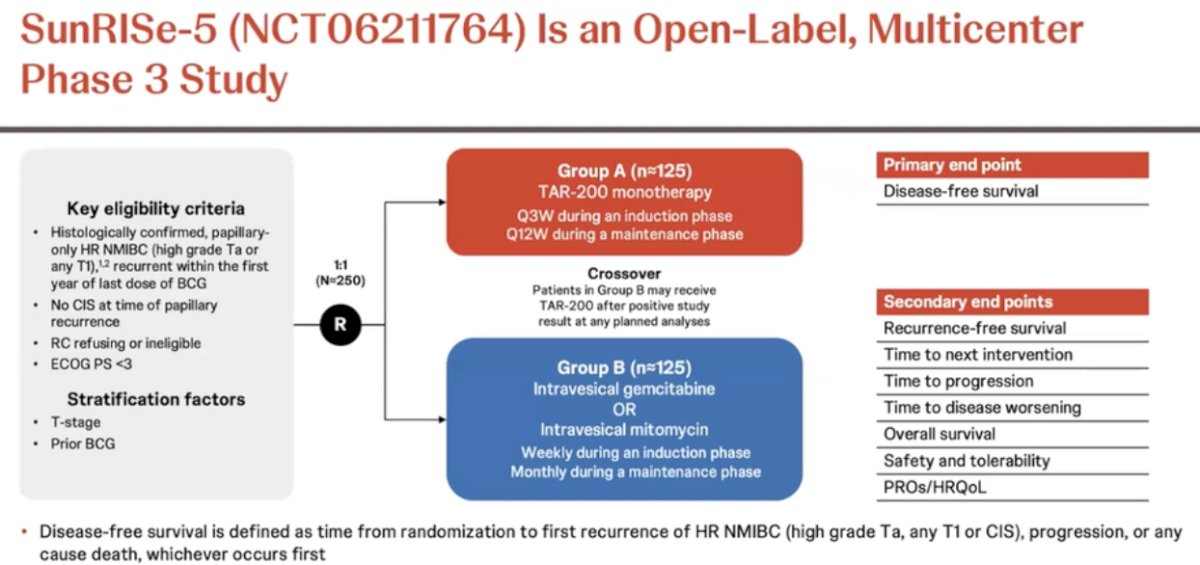
The time is now for a randomized trial design. There are multiple FDA-approved standards of care’ available now, and any of these drugs could act as a control arm in randomized comparison trials. In this scenario, all patients in such trials would receive (potentially) active drugs. Dr. Meeks argued that a 2:1 randomization is likely the way to go in such trials. An example of such a trial is SunRISe-5, which is a randomized, open-label, multicenter phase III trial that is evaluating the safety and efficacy of TAR-200 compared with investigator’s choice of intravesical chemotherapy in patients with papillary-only, high-risk NMIBC that recurs within the first year of BCG treatment who either refuse or are ineligible for radical cystectomy. In this trial, eligible patients (n=250) will undergo 1:1 randomization to either:
- TAR-200 monotherapy given every 3 weeks during induction and every 12 weeks during the maintenance phase
- Intravesical gemcitabine or intravesical mitomycin (weekly during induction, monthly during maintenance)
Illustrated below is Dr. Meeks’ proposed framework for trial design of drugs for BCG unresponsive NMIBC. The investigational drug would be compared to a standard of care Drug B. All patients would be required to undergo blue light cystoscopy with mapping biopsies both at 3 and 12 months. The primary endpoints would be durability (more meaningful in this setting), with a secondary endpoint of complete response.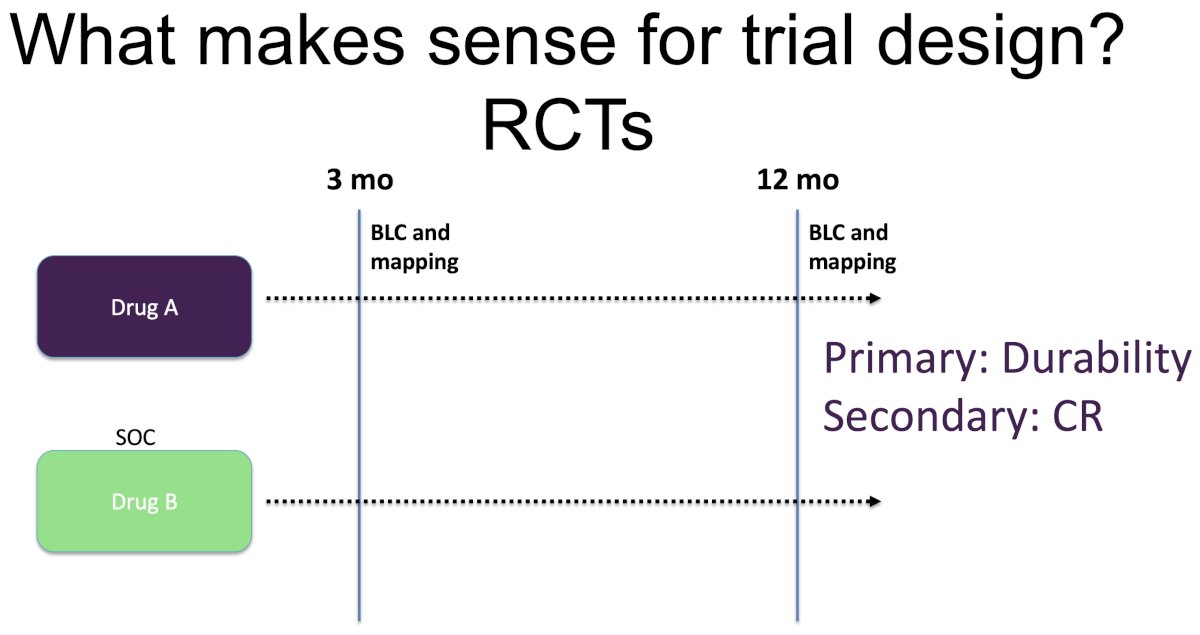
Can we take this even further and adopt STAMPEDE-like platform?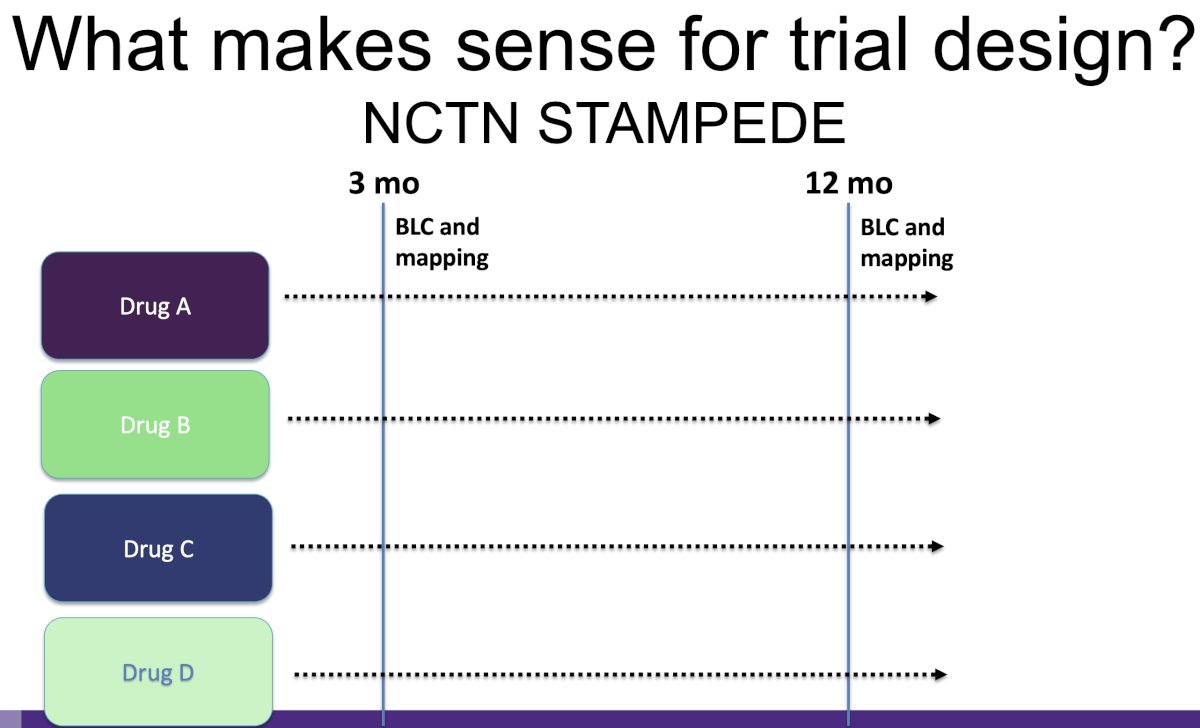
Some important measures to mitigate bias in single arm trials include:
- Mandating central pathology review
- Use of standardized cystoscopy (potentially recorded)
- Mandating mapping biopsies at set intervals
- Decreasing the heterogeneity of patients
- Using clinically meaningful endpoints such as 12 months durability
Dr. Meeks concluded with the following take home messages:
- The time is now for randomized controlled trials for drugs in the BCG unresponsive CIS disease space
- Single arm trials have been successful in leading to drug approvals in this space. However, they may lack utility moving forward given the existence of numerous standards of care now in this disease space.
- Funding support from pharmaceutical companies and entities such as the NIH will be crucial moving forward
Presented by: Joshua Meeks, MD, PhD, Associate Professor of Urology, Department of Urology, Northwestern University Feinberg School of Medicine, Chicago, IL
Written by: Rashid K. Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX between December 3rd and 6th, 2024
References:- Boorjian SA, Alemozaffar M, Konety BR, et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: A single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. 2020: S1470-2045(20)30540-4.
- Jarow JP, Lerner SP, Kluetz PG, et al. Clinical trial design for the development of new therapies for nonmuscle-invasive bladder cancer: report of a Food and Drug Administration and American Urological Association public workshop. Urology. 2014;83(2):262–4.
- Kamat AM, Sylvester RJ, Bohle A, et al. Definitions, End Points, and Clinical Trial Designs for Non-Muscle-Invasive Bladder Cancer: Recommendations From the International Bladder Cancer Group. J Clin Oncol. 2016; 34(16):1935-44.
- Myers AA, Shen Tan W, Grajales V, et al. Challenging the Paradigm of "BCG-unresponsive" Bladder Cancer: Does Additional Bacillus Calmette-Guérin Have an Effect? Eur Urol. 2024; 86(4):366-8.


