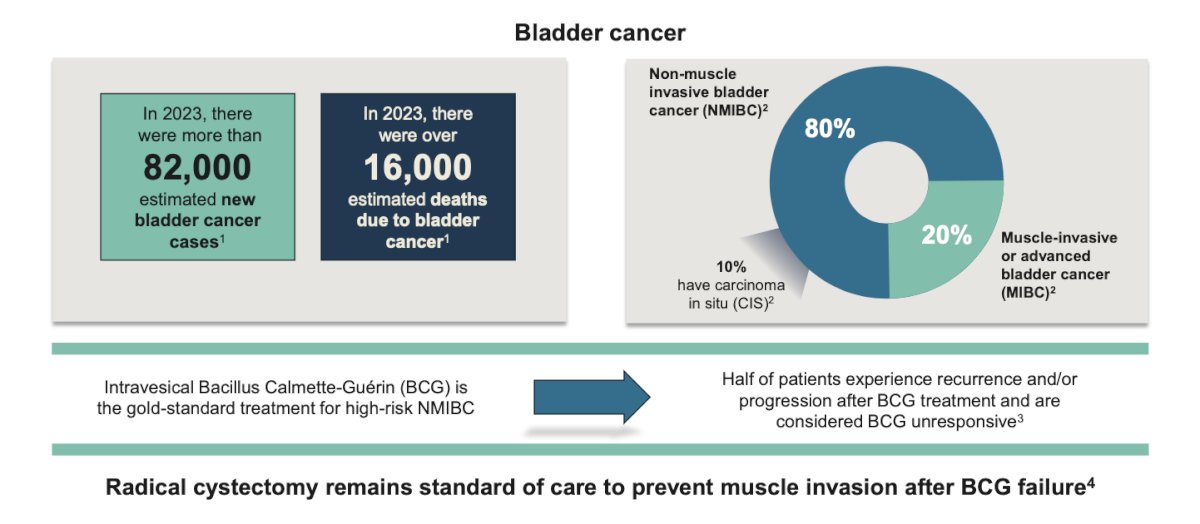
(UroToday.com) The 2024 SUO annual meeting included a bladder cancer session, featuring a presentation by Dr. Anthony Cheung discussing the mechanism of action and translation to the clinic of detalimogene voraplasmid (EG-70), a novel, investigational non-viral immunotherapy for non muscle invasive bladder cancer (NMIBC). Bladder sparing therapies for BCG-unresponsive NMIBC address an important unmet clinical need:
Detalimogene voraplasmid (formerly known as EG-70) is a novel, investigational, non-integrating, non-viral gene therapy that was specifically designed to elicit local stimulation of anti-tumor immune response in the bladder while mitigating the risk of systemic toxicities from immune stimulation. Detalimogene voraplasmid is administered by intravesical instillation to eligible patients with NMIBC to drive bladder localized expression of innate (retinoic acid-inducible gene I [RIG-I] agonists) and adaptive (IL-12) immune regulators and remodel the tumor microenvironment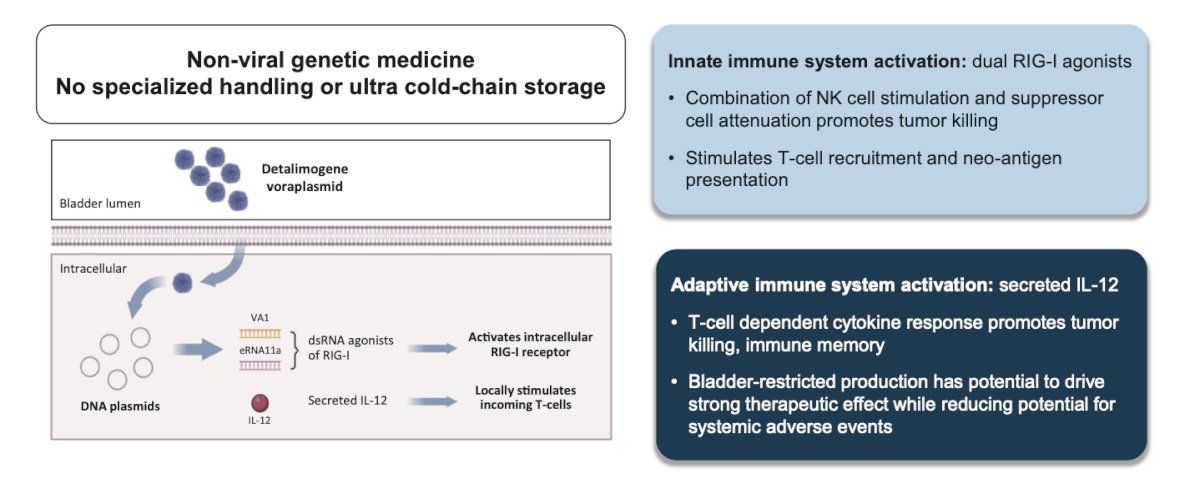
LEGEND is an ongoing phase 1/2 study investigating the safety and efficacy of detalimogene voraplasmid in patients with BCG-unresponsive NMIBC. At SUO 2024, Dr. Cheung and colleagues presented preclinical data supporting the mechanism of action of detalimogene voraplasmid, which involves immune cell recruitment, tumor microenvironment remodeling and, ultimately, immune training on neoantigens and tumor clearance.
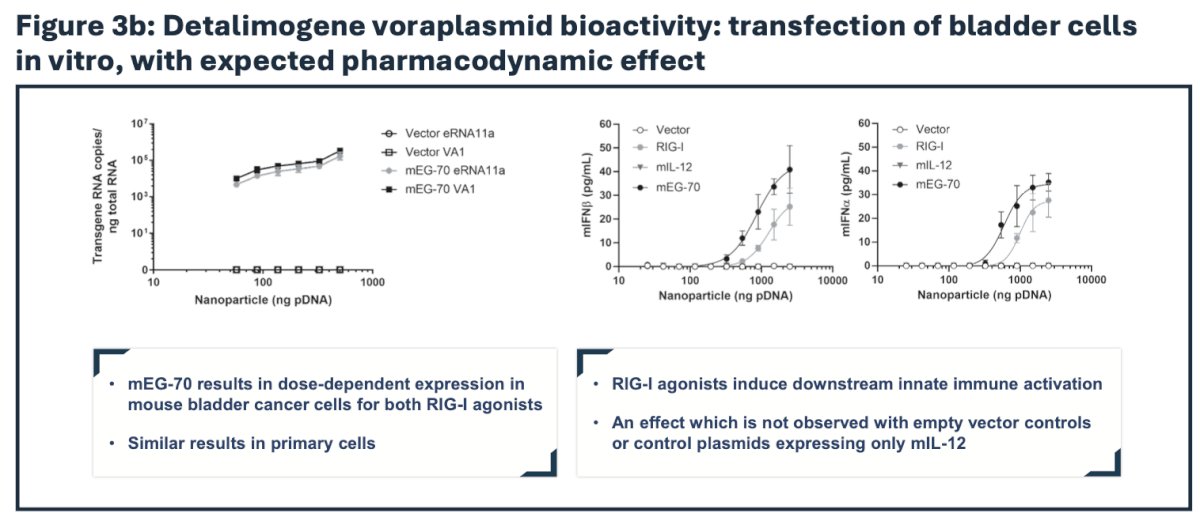
Preclinical evaluation of detalimogene voraplasmid efficacy was conducted in vitro in relevant cell lines and in an orthotopic syngeneic mouse model of bladder cancer to recapitulate a physiological tumor microenvironment in immunocompetent C57BL/6 mice. Luciferase-expressing MB49 cells were instilled in the bladder on study Day 1. Following confirmation of tumor engraftment by in vivo bioluminescence imaging on Day 9, mice received two weekly intravesical instillations of mEG-70 (a murine surrogate of EG-70) on Days 10 and 17. Efficacy of mEG-70 was assessed post-dosing by flow cytometry, MSD immunoassays, immunohistochemistry, bioluminescence in vivo imaging, bladder weight evaluation, and overall survival. In the phase 1 part of the LEGEND study, detalimogene voraplasmid was assessed in patients with high-risk BCG-unresponsive NMIBC with CIS.
Immune profiling by flow cytometry, immunoassay, and immunohistochemistry revealed a profound remodeling of the tumor microenvironment from an immunosuppressive phenotype to a pro-inflammatory milieu supportive of tumor clearance: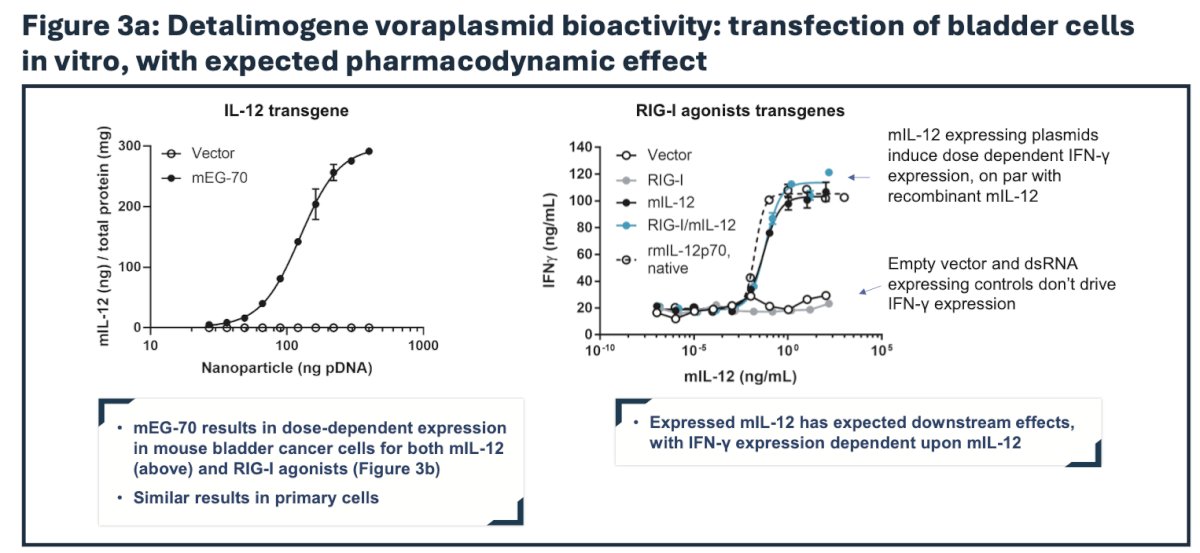
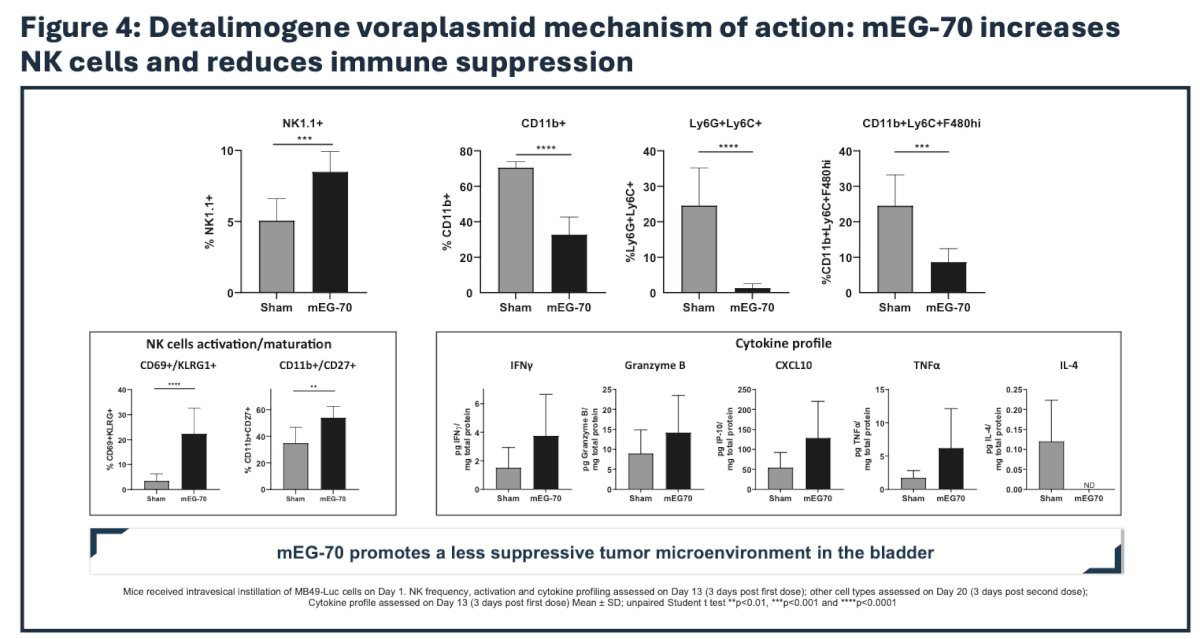
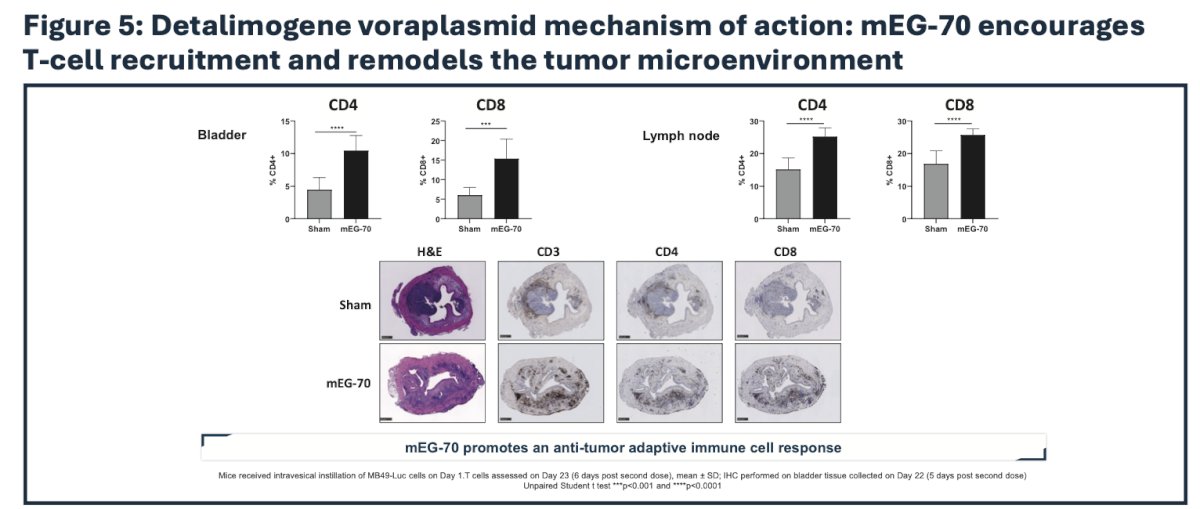
Accordingly, administration of mEG-70 was associated with marked reduction in tumor burden and marked improvement in survival in mice: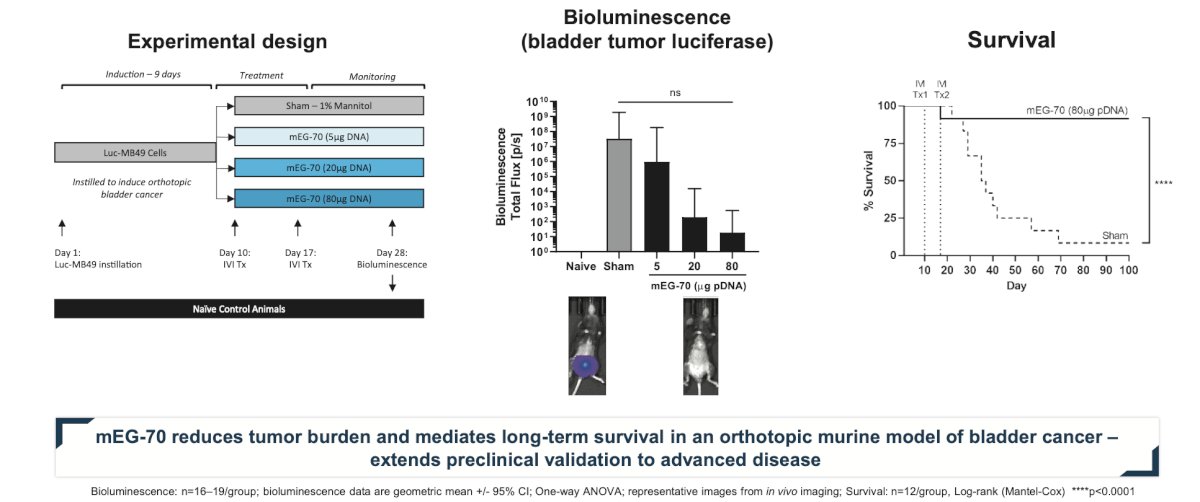
As demonstrated by either bladder or flank tumor cell rechallenge, the anti-tumor immune response in surviving tumor-free mice resulted in durable protection against subsequent tumor re-challenge, demonstrating systemic immune memory: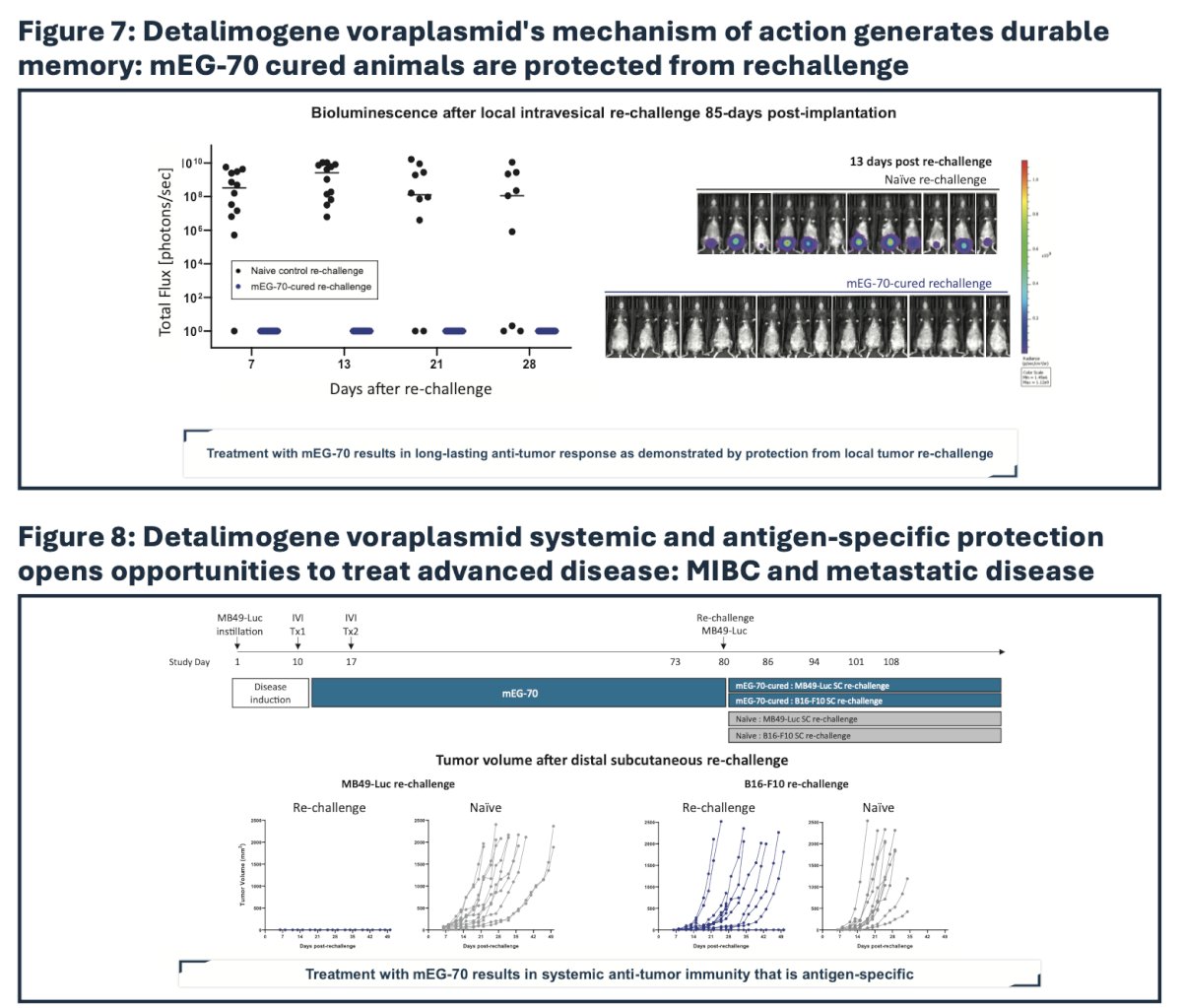
LEGEND is an ongoing, multicohort phase 1/2 study of detalimogene voraplasmid in patients with high risk NMIBC. Phase 1 patients include BCG-unresponsive NMIBC with CIS with detalimogene voraplasmid dosing 2 or 4 doses over a 12 week cycle. The cohorts are a 3+3 dose escalation, with 3 dose levels (0.25 ng/mL, 0.8 mg/mL, 2.5 mg/mL) and two schedules (2 doses/12-week cycle versus 4 doses/12 week cycle). The primary endpoint is safety and the secondary endpoint is efficacy at 3 months. Enrollment for phase 1 is now complete and treatment with detalimogene voraplasmid resulted in a 73% complete response rate at any time, and a promising safety and tolerability profile. The safety and efficacy findings from phase of LEGEND are summarized as follows: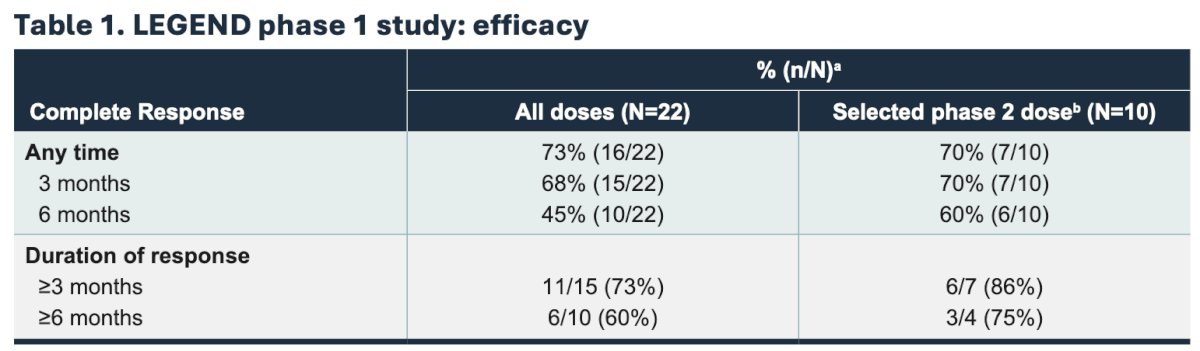
Dr. Cheung concluded his presentation discussing the mechanism of action and translation to the clinic of detalimogene voraplasmid with the following take home messages:
- Preclinical data indicate that:
- mEG-70 treatment results in robust activation of both innate and adaptive immune responses
- In tumor-bearing animals, mEG-70 remodels the tumor microenvironment resulting in clearance of existing tumors, with a durable memory-mediated protective effect
- Translation to the LEGEND clinical study:
- The phase 1 portion of LEGEND suggests a promising safety, tolerability, and efficacy profile
- 73% of patients achieve a complete response rate at any time, and at the dose selected for phase 2, complete response rates were 70% at 3 months and 60% at 6 months
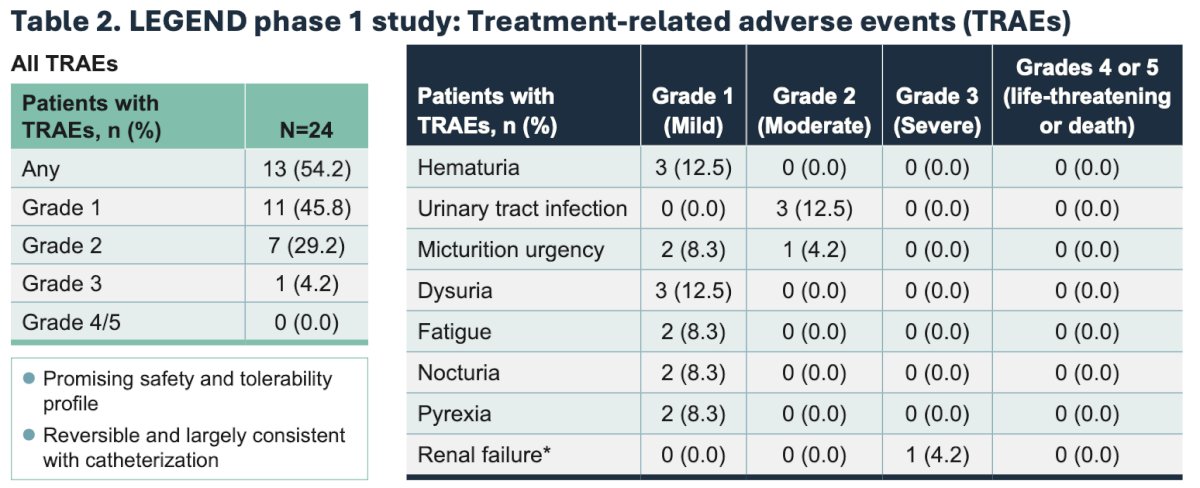
- Treatment related adverse events reported to date are mostly grade 1/2 and consistent with catheterization/intravesical administration.
- Phase 2 sites are currently being recruited and activated and patients are being enrolled.
Presented by: Anthony Cheung, CSO, enGene Inc., St-Laurent, Quebec, Canada
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Society of Urologic Oncology (SUO) Annual Meeting, Dallas, TX, Tues, Dec 3 – Fri, Dec 6, 2024.


