(UroToday.com) The 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX between December 3rd – 6th, 2024 was host to a bladder cancer poster session. Ian McElree presented the results of an analysis of the efficacy of salvage BCG for recurrent non-muscle invasive bladder cancer (NMIBC) following 1st line gemcitabine plus docetaxel.
BCG remains the recommended 1st line intravesical option for the adjuvant treatment of select intermediate risk and all high risk NMIBC patients following a complete transurethral resection of bladder tumor (TURBT). During the ongoing BCG shortage, intravesical gemcitabine plus docetaxel has emerged as a promising alternative for these patients, with prospective studies ongoing currently (i.e., EA8212 BRIDGE; NCT05538663).1
With the increased uptake of gemcitabine plus docetaxel, questions have emerged regarding the optimal sequencing of intravesical therapies, as well as the efficacy of BCG in the salvage setting. To date, the efficacy of intravesical BCG following intravesical gemcitabine plus docetaxel for recurrent NMIBC remains unknown. In this study, the authors sought to evaluate the efficacy of BCG for NMIBC patients with disease recurrence following intravesical gemcitabine plus docetaxel.
This was a retrospective analysis of 19 NMIBC patients treated at The Iowa Medical Center between January 2018 and April 2024, who received intravesical BCG following gemcitabine plus docetaxel failure. After complete TURBT, patients received a 6 weekly induction regimen of one vial of BCG (with or without 50 million units of IFNα-2b and/or interleukin 2). Maintenance therapy was initiated if patients were disease free at first follow-up. The study outcomes of interest were recurrence-free (RFS) and high-grade recurrence-free survivals (HG-RFS). Survival probabilities were estimated and plotted using Kaplan-Meier curves. The index date (i.e., T0) for both RFS and HG-RFS was defined as the start date of BCG induction. Recurrence was defined as tumor relapse in the bladder or prostatic urethra in males.The median study follow-up was 27 months. The median patient age was 67 years. All patients were treatment-naïve prior to gemcitabine plus docetaxel induction. Prior to BCG induction, 16/19 (84%) patients had high-grade disease, including 6 with (32%) carcinoma in situ (CIS). Nine (47%) and three (15%) patients received IFN-α and IL-2 concurrently with BCG induction, respectively. The median time to gemcitabine plus docetaxel failure was 8 months.

The 1- and 2-year RFS rates were 44% and 36%, respectively.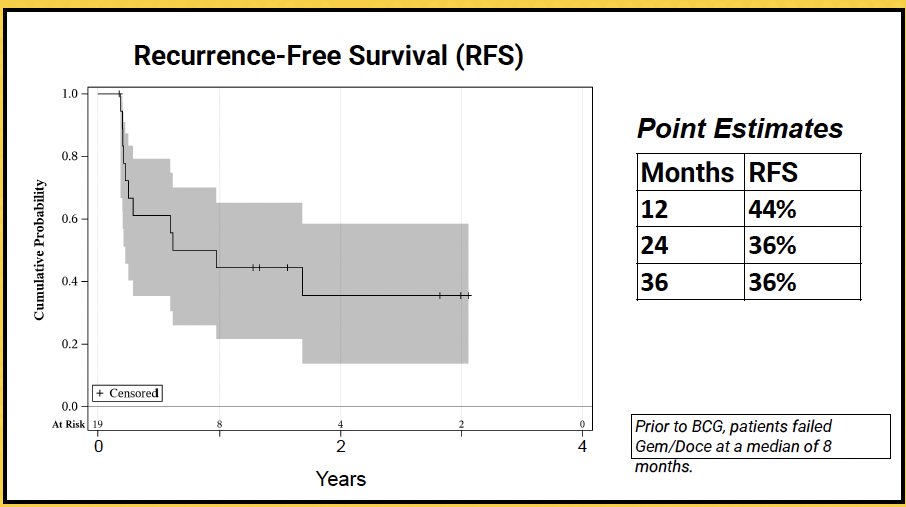
HG-RFS in the cohort of patients with high-grade disease prior to gemcitabine plus docetaxel was 53% at 1 year.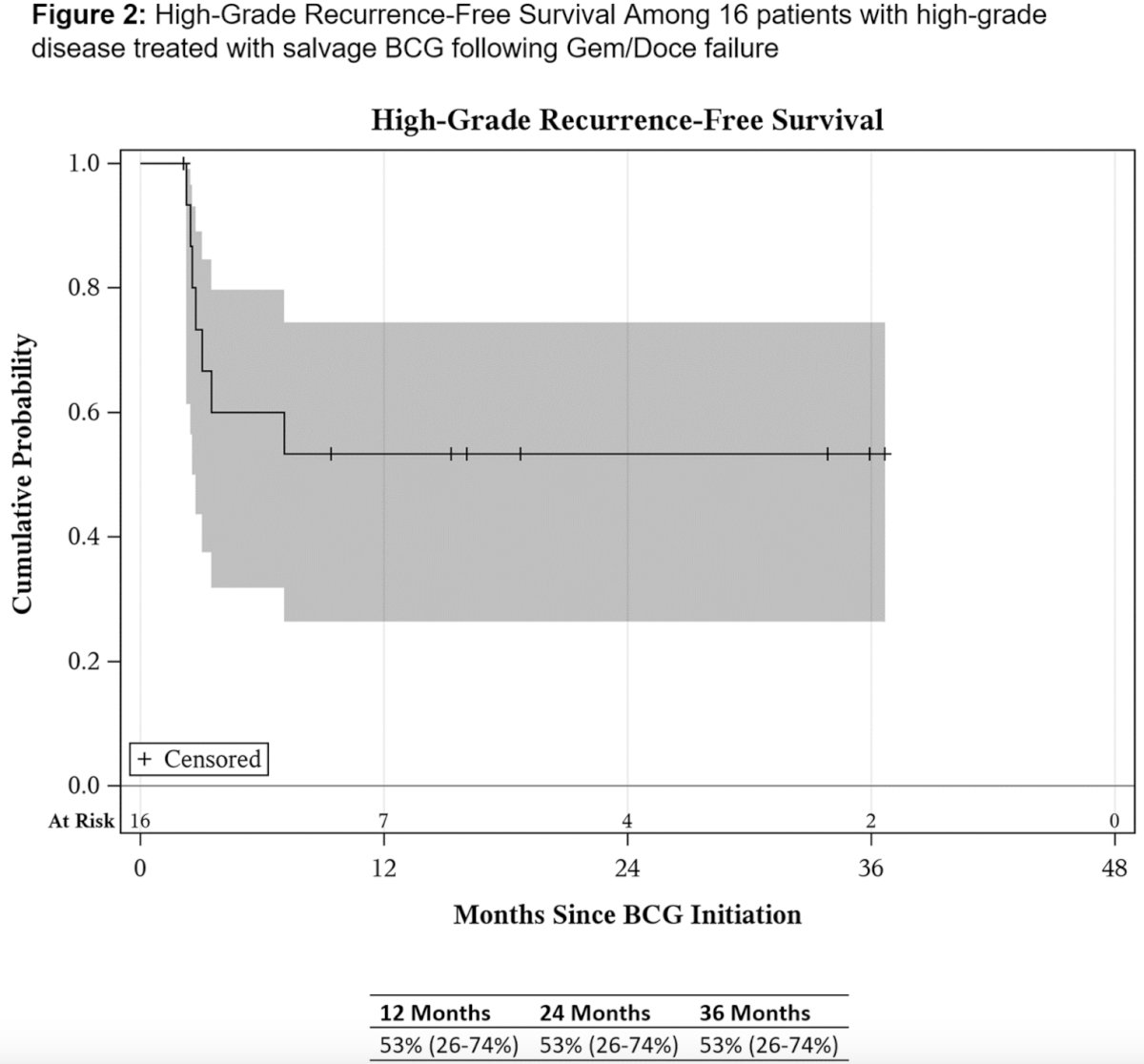
Two patients ultimately elected for cystectomy with pathology of pT2N0 and pT1N0. One patient developed metastatic urothelial carcinoma despite a lack of muscle invasive disease. One patient was unable to finish a full BCG induction due to side effects.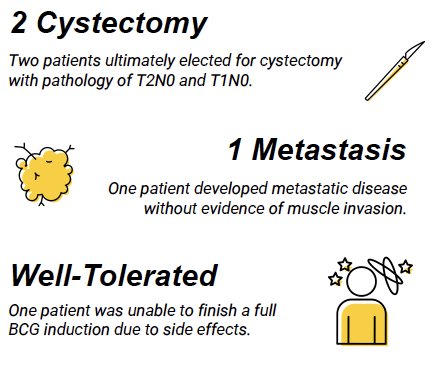
Dr. McElree concluded that in a cohort of patients with recurrent NMIBC, intravesical BCG was a reasonable treatment option following gemcitabine plus docetaxel failure. Larger studies are further needed.
Presented by: Ian M. McElree, Medical Student, University of Iowa Roy J. and Lucille A. Carver College of Medicine, Iowa City, IA
Written by: Rashid K. Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX between December 3rd and 6th, 2024
References:

