(UroToday.com) The 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX between December 3rd – 6th, 2024 was host to a bladder cancer poster session. Dr. Sima Porten presented SunRISe-5, a phase III randomized, open-label study of TAR-200 compared with intravesical chemotherapy after bacillus Calmette-Guerin (BCG) in recurrent, high-risk, non-muscle invasive bladder cancer (NMIBC).
A significant proportion of patients with high-risk NMIBC recur following intravesical BCG therapy (20–46%)1-2. Additional BCG is not effective for patients with early recurrences (i.e., within 1 year) and is not currently recommended by guidelines in this setting. Treatment options for such patients remain limited. Currently, the standard-of-care for early BCG-unresponsive recurrences of papillary-only high-risk NMIBC remains radical cystectomy. This is an area of high unmet need, and there are ongoing efforts to develop bladder-sparing, localized treatments for patients with papillary-only recurrent high-risk NMIBC, given that recently approved treatment options for BCG-unresponsive NMIBC are limited to patients with CIS.
TAR-200 is a novel drug delivery system for the sustained local release of gemcitabine in the bladder, relying on an osmotic system for sustained release as illustrated below. Evaluation of urine and plasma gemcitabine concentrations over a 7-day period have demonstrated the ability of TAR-200 to provide sustained, local delivery of gemcitabine while limiting systemic toxicity. As demonstrated in the figure below, intravesical instillation of gemcitabine (blue curve) is associated with a sharp increase/decrease in the gemcitabine urine concentration, which is non-sustained beyond day 1. Conversely, we see an increase in the urine gemcitabine concentration between days 1 and 3 with TAR-200 (red curve), followed by a gradual decline with measurable levels detected until at least day 7. Significantly, no gemcitabine was detected in the plasma of patients receiving TAR-200.
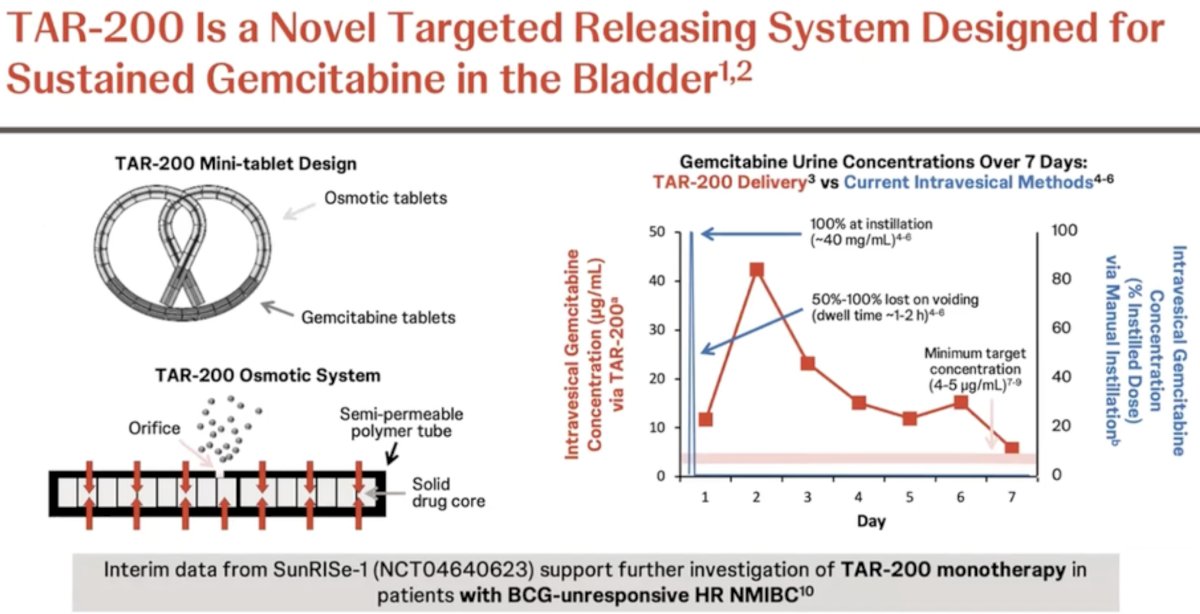
TAR-200 has been evaluated in SunRISe-1 (NCT04640623), which demonstrated promising results for TAR-200 monotherapy in patients with BCG-unresponsive, high-risk CIS (+/- papillary disease), with a 48-week complete response rate of 73%, supporting further investigation of TAR-200 monotherapy in other cohorts of patients with high-risk NMIBC. 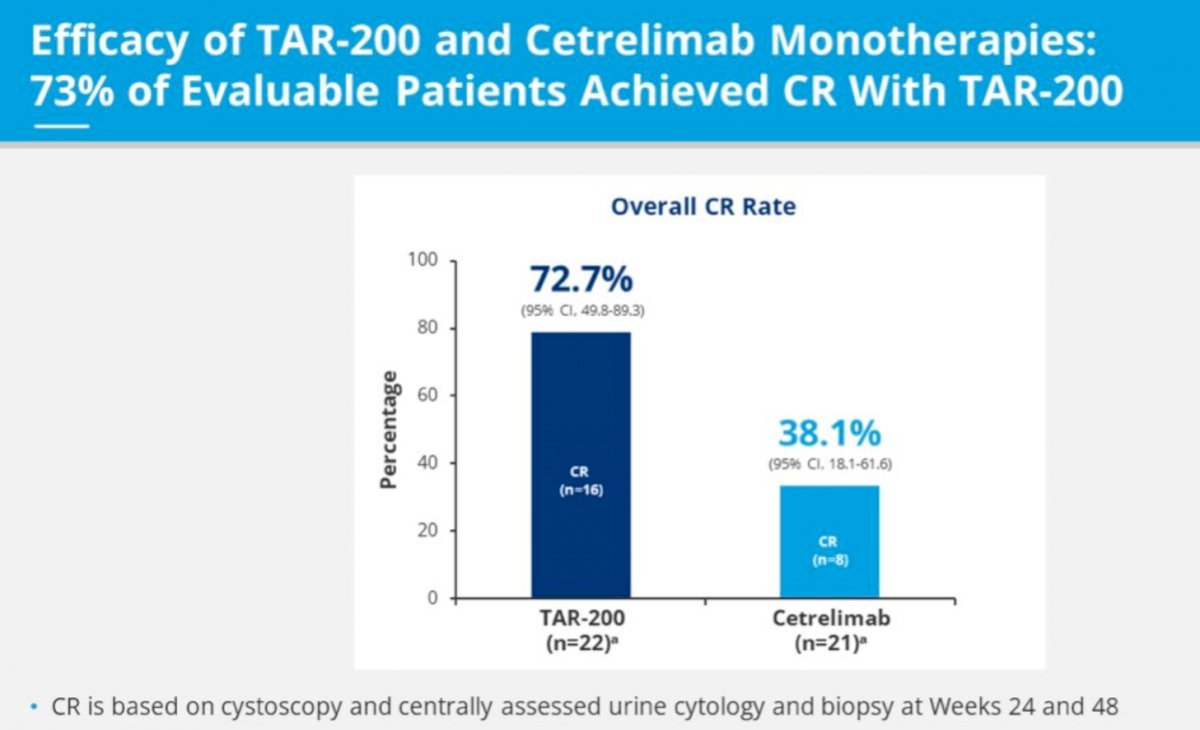
SunRISe-5 (NCT06211764) is a randomized, open-label, multicenter phase III trial that is evaluating the safety and efficacy of TAR-200 compared with investigator’s choice of intravesical chemotherapy in patients with papillary-only, high-risk NMIBC that recurs within the first year of BCG treatment who either refuse or are ineligible for radical cystectomy.
The study design is illustrated below. Eligible patients are those aged ≥18 years, have ECOG performance status of 0–2, and were diagnosed ≤90 days of informed consent with histologically confirmed recurrent, papillary-only high-risk NMIBC (high-grade Ta, any T1, without carcinoma in situ) with last dose of BCG ≤12 months, and who are ineligible for or decline radical cystectomy. Eligible patients (n=250) will undergo 1:1 randomization to either:
- TAR-200 monotherapy given every 3 weeks during induction and every 12 weeks during the maintenance phase
- Intravesical gemcitabine or intravesical mitomycin (weekly during induction, monthly during maintenance)
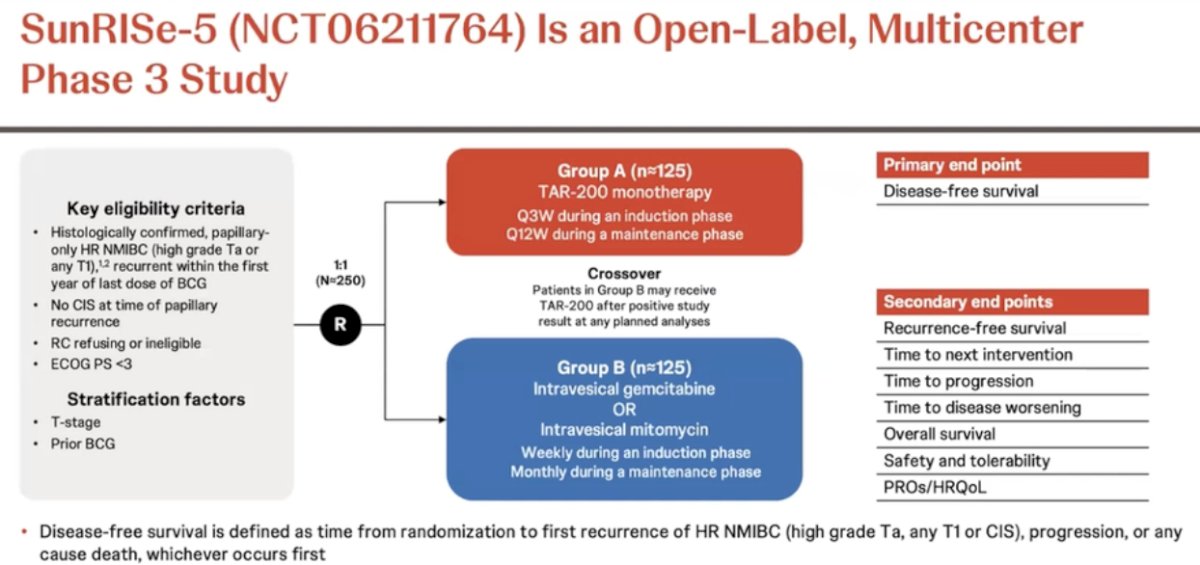
The primary endpoint is disease-free survival, defined as time from randomization to first recurrence of high-risk NMIBC (high grade Ta, any T1, or CIS). The secondary endpoints are as follows:
- Recurrence-free survival
- Time to next intervention
- Time to progression
- Time to disease worsening
- Overall survival
- Safety and tolerability
- Patient-reported outcomes
- Health-related quality of life
SunRISe-5 is currently recruiting in Argentina, Belgium Brazil, China, France, Germany, Italy, Japan, Mexico, Poland, Romania, South Korea, Spain, the United Kingdom, and the United States. As of August 1, 2024, 25 patients have been randomized, and recruitment is ongoing at 106 sites.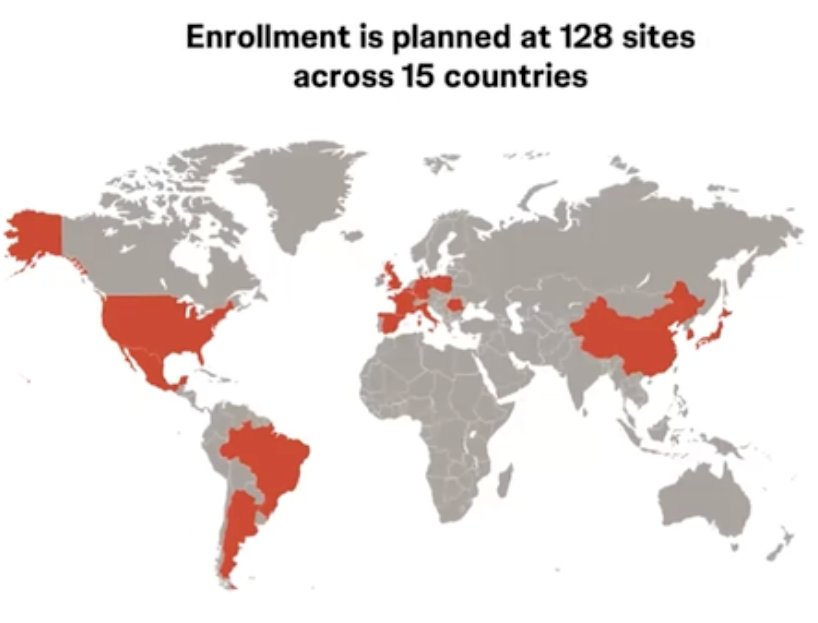
Presented by: Sima Porten, MD, MPH, Assistant Professor, Department of Urology, University of California, San Francisco, CA
Written by: Rashid K. Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX between December 3rd and 6th, 2024
References:- Matulay JT, Li R, Hensley PJ, et al. Contemporary Outcomes of Patients with Nonmuscle-Invasive Bladder Cancer Treated with bacillus Calmette-Guérin: Implications for Clinical Trial Design. J Urol. 2021; 205(6):1612-21.
- Contieri R, Hensley PJ, Tan WS, et al. Oncological Outcomes for Patients with European Association of Urology Very High-risk Non-muscle-Invasive Bladder Cancer Treated with Bacillus Calmette-Guérin or Early Radical Cystectomy. Eur Urol Oncol. 2023; 6(6):590-6.


