(UroToday.com) The 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX between December 3rd – 6th, 2024 was host to a bladder cancer poster session. Dr. Ahmed Eraky presented the results of an analysis demonstrating that low- and high-grade intermediate-risk non-muscle invasive bladder cancer (NMIBC) respond differently to Bacillus Calmette-Guérin (BCG) and gemcitabine + docetaxel.
Given the ongoing BCG shortage worldwide, the intravesical combination of gemcitabine + docetaxel has emerged as a practical, efficacious alternative to BCG for the adjuvant treatment of select intermediate- and high-risk NMIBC patients following a complete transurethral resection of bladder tumor (TURBT).1 The intermediate-risk group is heterogeneous and includes patients with both low- and high-grade tumors. As such, further evaluation of the efficacy of gemcitabine + docetaxel in the intermediate risk group, stratified by tumor grade, is needed. The objective of this study was to compare the efficacy of gemcitabine + docetaxel to BCG for treating low- and high-grade intermediate-risk NMIBC.
This was a single center, retrospective analysis of intermediate-risk NMIBC patients who received either induction intravesical BCG or gemcitabine + docetaxel between 2013 and 2024. Maintenance regimens were administered to patients without evidence of recurrence following induction therapy. Follow-up was performed in accordance with the AUA guidelines. The study outcomes of interest were recurrence-free (RFS) and progression-free survivals (PFS). Time-to-event analyses were performed using Kaplan-Meier curves.
Of the 483 evaluable patients, 127 had intermediate-risk disease (high grade: 83; low grade: 44). Of the 83 patients with high grade disease, 44/83 received induction BCG and 39/83 received induction gemcitabine + docetaxel. Conversely, for the 44 low grade intermediate-risk tumors, induction BCG and gemcitabine + docetaxel were administered to 22 patients each. The median patient age ranged between 68 and 71 years. The median study follow-up ranged between 28 and 34 months.
The overall 2-year any grade RFS rate was 52% (95% CI: 43–63%). The 2-year any grade RFS rates by underlying grade were as follows:
- High grade: 55% (95% CI: 44–68%)
- Low grade: 47% (95% CI: 33–66%)
- Log rank p=0.093
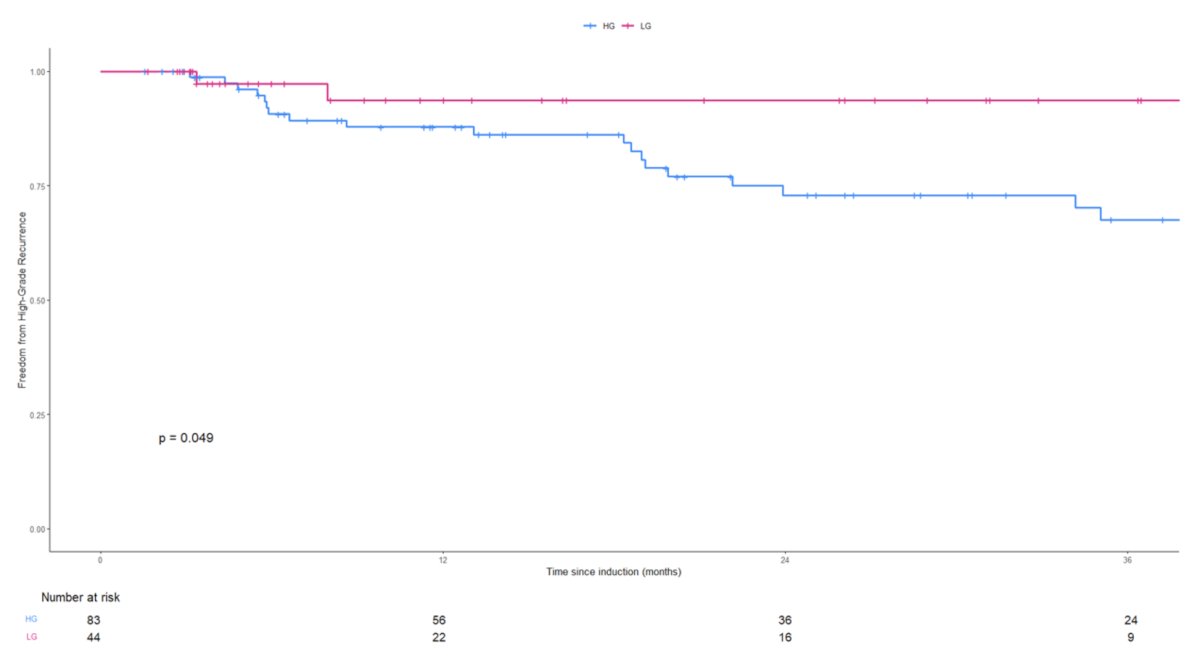
The corresponding 2-year high grade RFS rates were as follows:
- High grade: 58% (95% CI: 63 – 85%)
- Low grade: 94% (95% CI: 86–100%)
- Log rank p=0.049
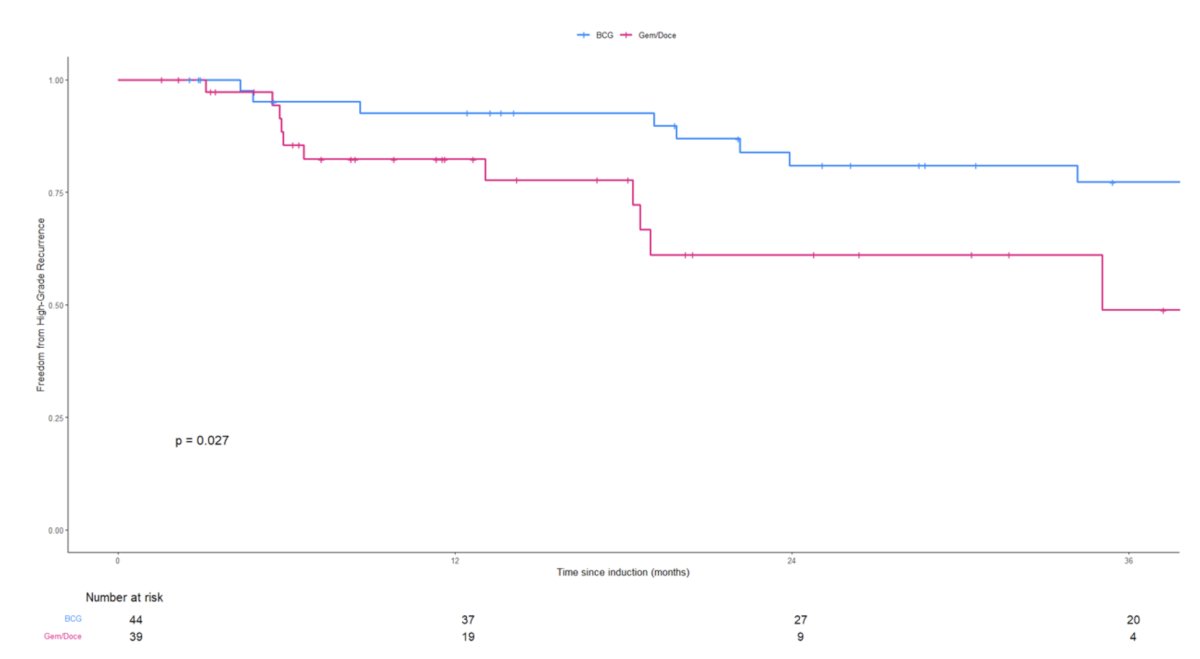
Among patients in the high-grade subgroup, the 2-year high-grade RFS rates were superior for those who received BCG (81% versus 61% for gemcitabine + docetaxel; log-rank p=0.027).
Among patients in the low-grade subgroup, the corresponding 2-year high-grade RFS rates were 89% and 100% for BCG and gemcitabine + docetaxel-treated patients, respectively (log-rank p=0.22).
Based on these results, Dr. Eraky and colleagues concluded that BCG is associated with superior high-grade RFS, compared to gemcitabine + docetaxel, for high-grade, intermediate risk NMIBC patients. Conversely, there were no differences in the 2-year high-grade RFS rates between the two intravesical treatment groups for low grade, intermediate risk patients. Prospective randomized multi-center studies are needed to investigate and further personalize the intravesical adjuvant therapy regimen in the intermediate risk NMIBC group and possibly modify risk stratification groups to separate low- and high-grade tumors.
Presented by: Ahmed Eraky, MBBCh, FEBU, Society of Urologic Oncology (SUO) Fellow, Mount Sinai Hospital, New York, NY
Written by: Rashid K. Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX between December 3rd and 6th, 2024
References:

