(UroToday.com) The 2024 SUO annual meeting included a urothelial carcinoma session, featuring a presentation by Dr. Sandip Prasad discussing the ENVISION trial assessing primary chemoablation of recurrent low-grade intermediate risk NMIBC with UGN-102. Low-grade, intermediate-risk NMIBC is a persistent and recurrent cancer that is inadequately controlled by the current standard of care, TURBT.
Recent studies have shown that many patients with low-grade intermediate risk NMIBC can be successfully treated with UGN-102, a reverse thermal gel containing mitomycin administered via intravesical instillation in the outpatient setting, removing the need for surgery.1,2 At SUO 2024, Dr. Prasad and colleagues reported efficacy and safety data from ENVISION, an ongoing prospective, phase 3, multinational, single-arm trial, designed to evaluate UGN-102 as primary chemoablative therapy in patients with a history of recurrent low-grade intermediate-risk NMIBC requiring TURBT.
Key inclusion criteria are as follows:
- History of >=1 prior episodes of low grade NMIBC requiring treatment with TURBT
- Low grade NMIBC (Ta) confirmed by cystoscopy and cold cup biopsy of the visualized tumor at screening or within 8 weeks before screening.
- Negative voiding cytology for high grade disease at screening or within 8 weeks before screening
- Intermediate risk disease, defined as 1 or 2 of the following: multiple tumors, longest tumor diameter >3 cm, early or frequent recurrence (ie. >=1 episode within the previous year)
Enrolled patients received 6 weekly intravesical instillations of UGN-102, a reverse thermal hydrogel containing mitomycin (75 mg). Approximately 3 months after the first instillation of UGN-102, patients underwent endoscopic evaluation, urine cytology testing, and for-cause biopsy to determine the presence or absence of bladder cancer. The primary endpoint was complete response (absence of bladder cancer) at the 3-month visit. Patients who achieved complete response entered the follow-up period. Patients not achieving complete response by the 3-month visit received standard of care treatment and then entered follow-up. The key secondary endpoint was duration of response, defined as the time from the date of evidence of complete response at the 3-month visit to the earliest date of disease recurrence, progression, or death from any cause, whichever occurred first. Safety outcomes were also evaluated: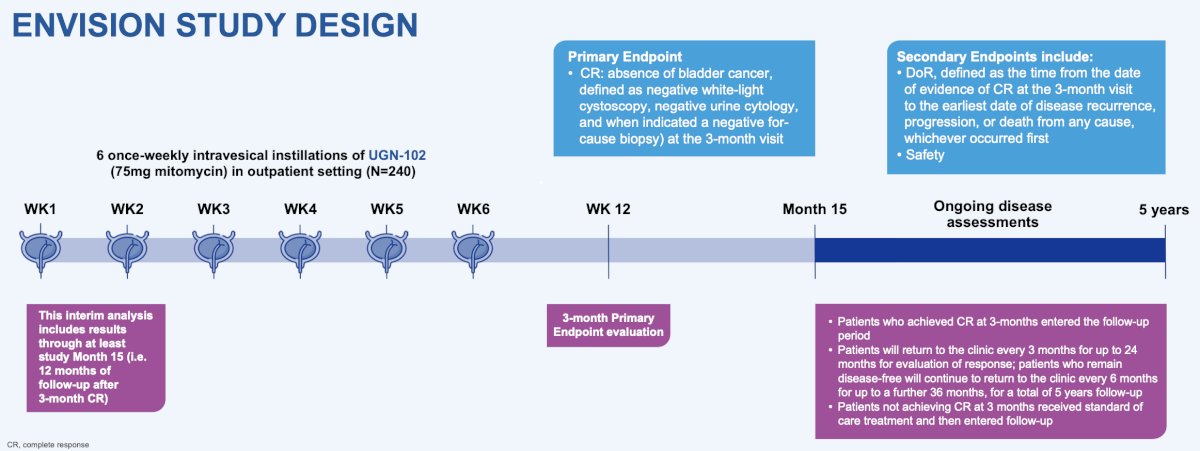
There were 240 patients with low-grade intermediate-risk NMIBC who met the eligibility criteria that were enrolled (61% male, 98% white, 68% aged ≥ 65 years). All enrolled patients received at least one dose of UGN-102, and 95% (n = 228) received all 6 weekly doses: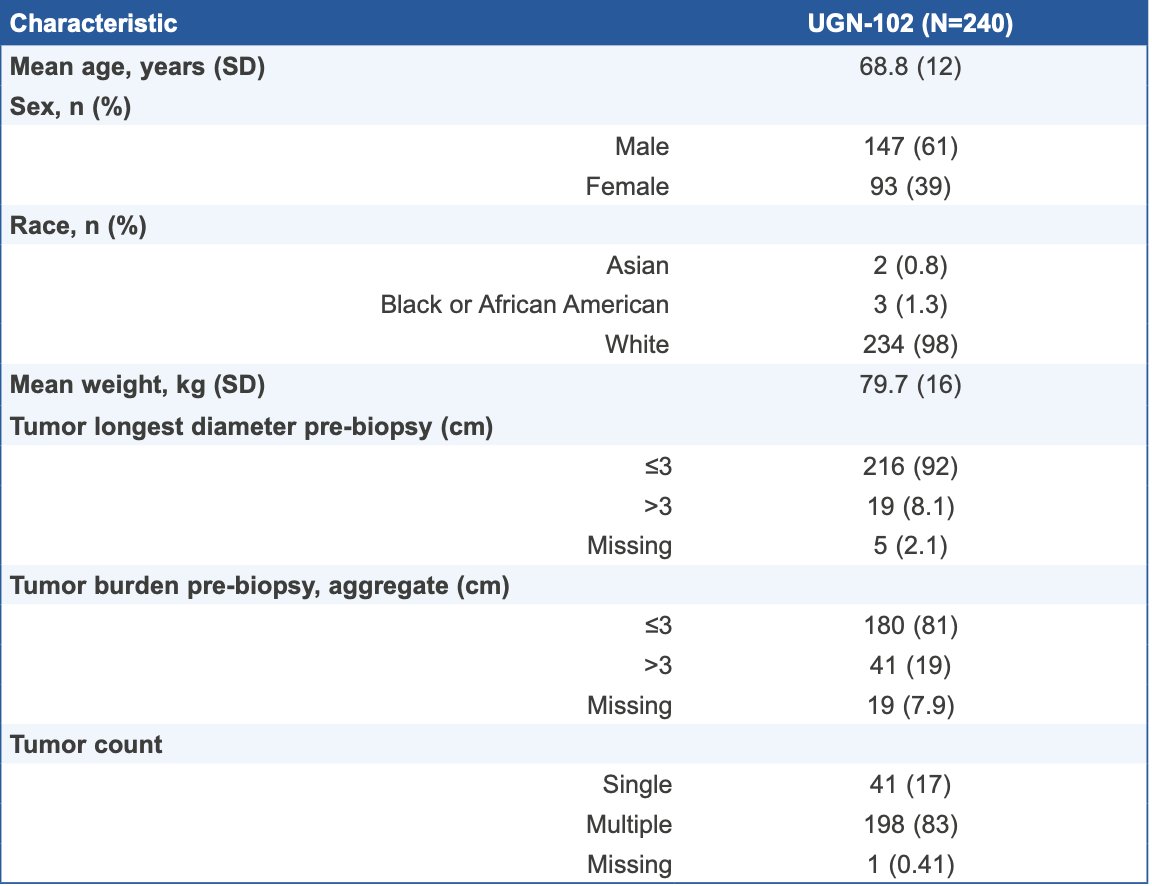
The primary endpoint of complete response at 3 months was achieved by 191 patients (79.6%; 95% CI 73.9–84.5):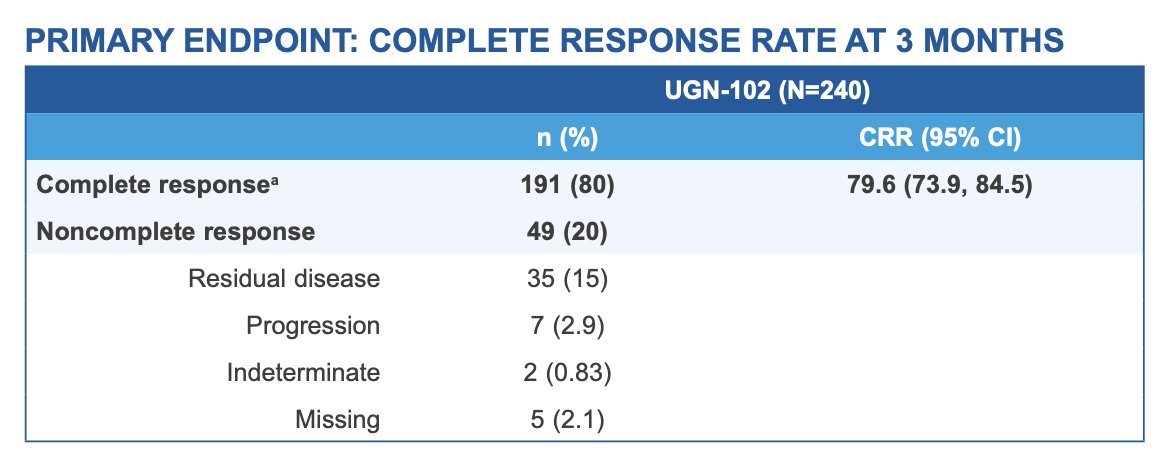
For these patients, the probability of remaining event-free at 12 months following the 3-month visit was 82.3% (95% CI 75.9–87.1) according to Kaplan–Meier estimate. The duration of response estimates at 15 months (n = 43) and 18 months (n = 9) months after 3 month complete response were both 80.9%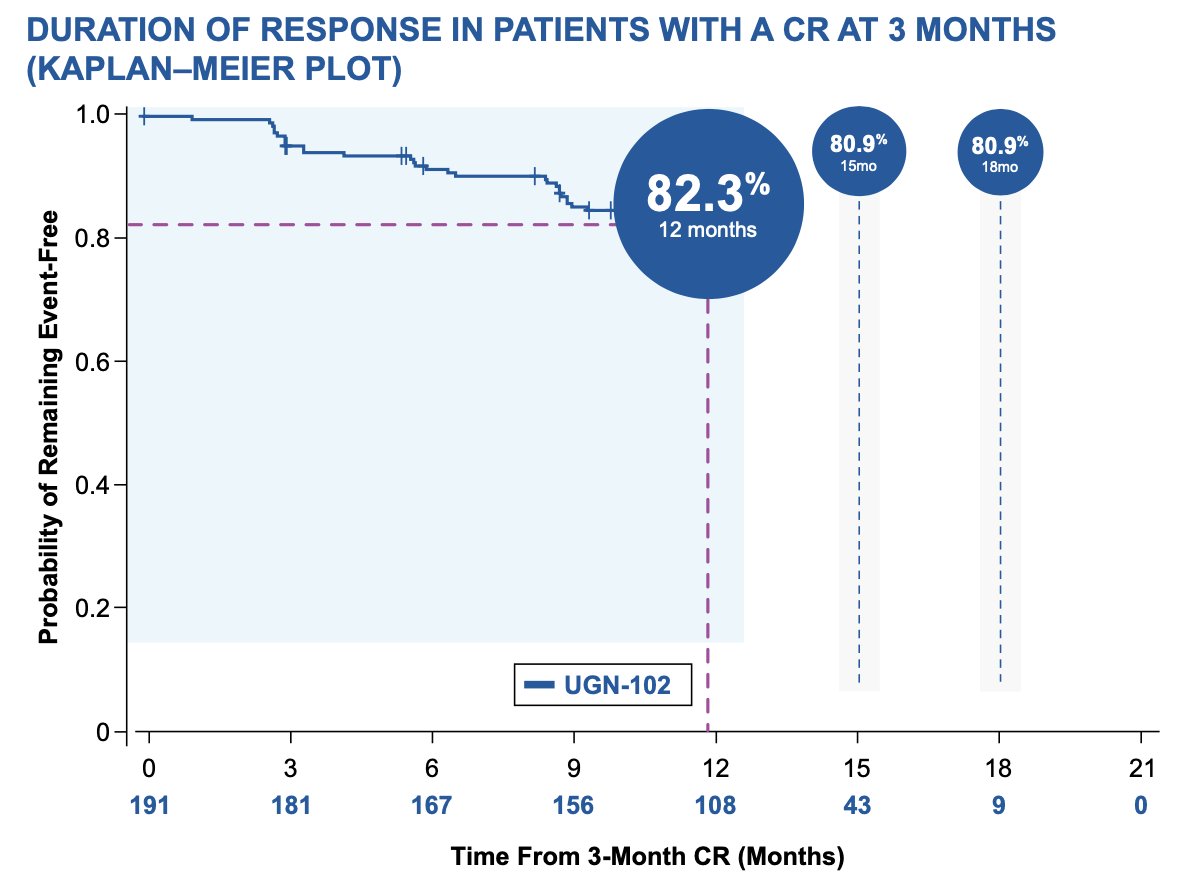
Median duration of response was not estimable over a median follow-up of 13.9 months. The most common adverse events (≥ 5.0% of patients), were dysuria, hematuria, urinary tract infection, pollakiuria, fatigue, and urinary retention. Adverse events were generally mild-to-moderate in severity and resolved or resolving. Serious adverse events occurred in 12% (29/240), two of which were considered treatment-related (urinary retention and urethral stenosis, both of which resolved):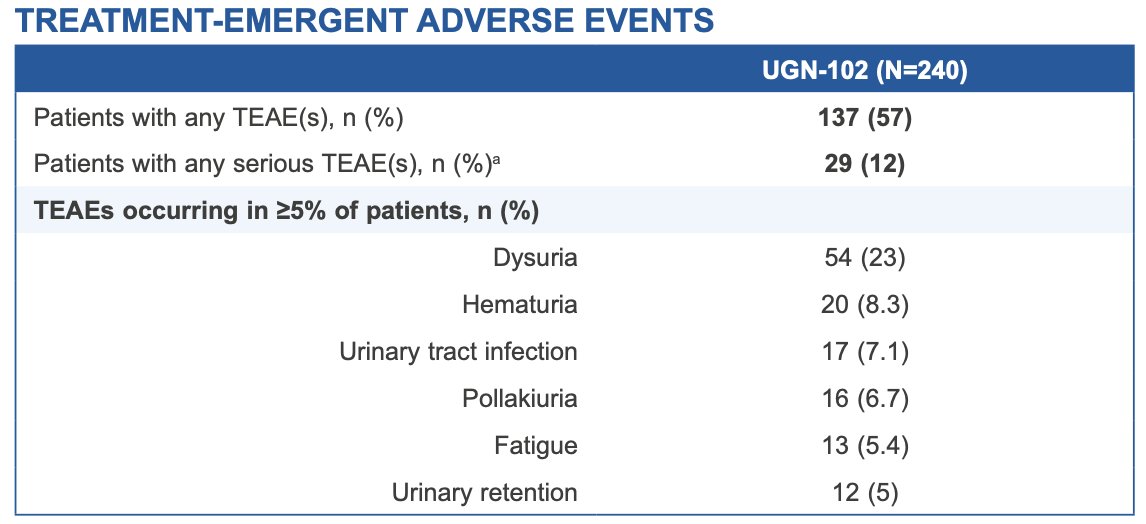
Dr. Prasad concluded his presentation discussing the ENVISION trial assessing primary chemoablation of recurrent low-grade intermediate risk NMIBC with UGN-102 with the following take home messages:
- Results from ENVISION demonstrate that primary chemoablation using UGN-102 results in a high and clinically meaningful complete response rate in patients with recurrent low-grade intermediate-risk NMIBC
- Furthermore, the durability of effect is robust, with trial participants who achieved an initial complete response having a high probability of remaining disease free 12 months later
- These observations provide further evidence that UGN-102 may represent a well-tolerated and valuable alternative to TURBT for patients with low-grade intermediate-risk NMIBC
Presented by: Sandip M. Prasad, MD, Morristown Medical Center/Atlantic Health System and Garden State Urology, Morristown, NJ
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Society of Urologic Oncology (SUO) Annual Meeting, Dallas, TX, Tues, Dec 3 – Fri, Dec 6, 2024.
References:
- Prasad SM, Huang WC, Shore ND, et al. Treatment of low-grade intermediate-risk nonmuscle-invasive bladder cancer with UGN-102 +/- transurethral resection of bladder tumor compared to transurethral resection of bladder tumor monotherapy: A randomized, controlled, phase 3 trial (ATLAS). J Urol. 2023 Oct;210(4):619-629.
- Prasad SM, Shishkov D, Mihaylov NV, et al. Primary chemoablation of recurrent low-grade intermediate-risk nonmuscle invasive bladder cancer with UGN-102: A single-arm, open-label, phase 3 trial (ENVISION). J Urol. 2024 Oct 24 [Epub ahead of print].


