(UroToday.com) The 2024 SUO annual meeting included a urothelial carcinoma session, featuring a presentation by Dr. Robert Svatek discussing PIVOT-006, a phase 3, randomized study of adjuvant intravesical cretostimogene grenadenorepvec versus surveillance for the treatment of intermediate-risk non muscle invasive bladder cancer (NMIBC).
AUA and NCCN Bladder Cancer Guidelines recommend adjuvant intravesical therapy or surveillance for patients diagnosed with intermediate risk NMIBC. Despite this, up to 30%-60% of patients will recur and there is a general lack of level 1 evidence in the management of intermediate risk NMIBC. As a result, there is both a knowledge gap and an unmet medical need for improved therapies in the adjuvant setting of intermediate risk NMIBC.
Cretostimogene grenadenorepvec is an oncolytic immunotherapy designed to selectively replicate in bladder cancer cells with Rb-E2F pathway alterations, commonly found in high-risk BCG-unresponsive NMIBC. In addition, cretostimogene also expresses GM-CSF adding to local and systemic cancer control: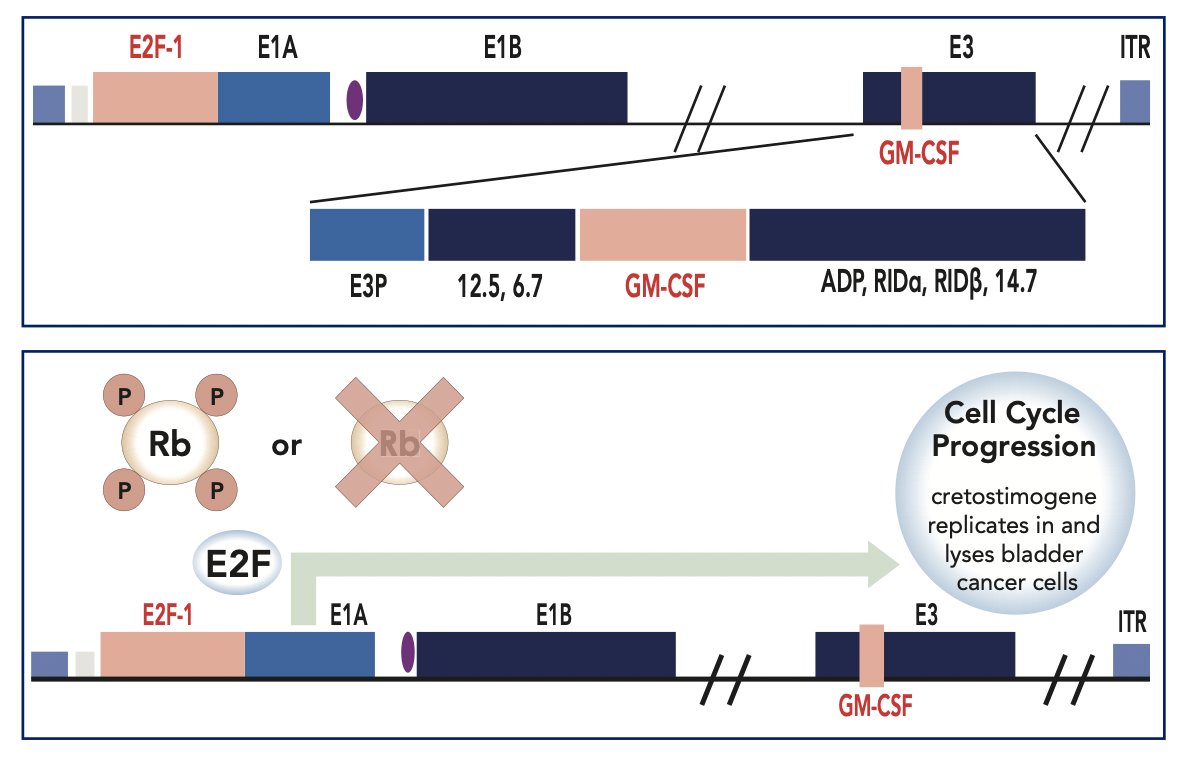
Cretostimogene received both US FDA Fast Track and Breakthrough Therapy Designations in the high risk, BCG-unresponsive NMIBC indication and has demonstrated a consistently favorable tolerability and safety profile. Based on the strength of those data, PIVOT-006 has been designed as a multi-national, randomized phase 3 study to assess the efficacy and safety of adjuvant cretostimogene versus surveillance in patients with intermediate risk NMIBC.
Eligibility criteria for PIVOT-006 include:
- Histologically confirmed intermediate risk NMIBC diagnosis within 90 days of randomization, as defined by AUA/SUO Guidelines
- Age ≥18 years
- ECOG performance status of 0-2
- Absence of nodal or metastatic disease at screening
Stratification factors include receipt of single-dose perioperative chemotherapy and tumor grade. Patients (~n = 364) will be randomized 1:1 to receive intravesical cretostimogene (Cohort 1) adjuvant to TURBT or surveillance (Cohort 2). Participants in Cohort 2 will be eligible to receive intravesical cretostimogene if recurrence with intermediate risk NMIBC is noted: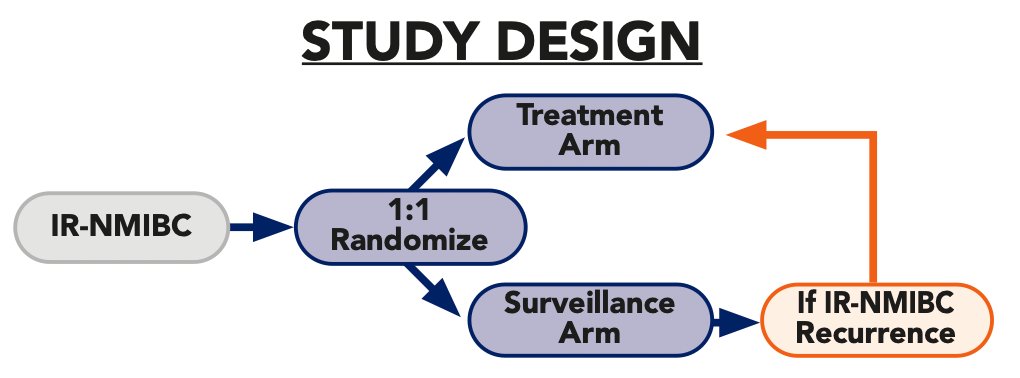
Intravesical cretostimogene will be instilled in combination with DDM, a transduction agent, for 6 weekly doses during the induction phase, followed by 3 weekly maintenance cycles at months 3 and 6, and culminating in single intravesical doses at months 9 and 12: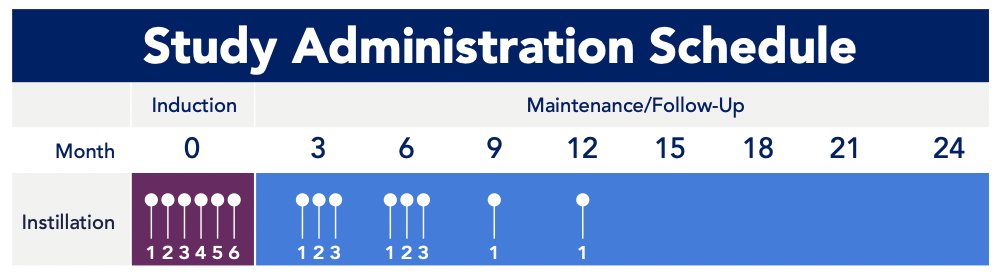
Primary disease assessments include serial cystoscopy, urine cytology, axial imaging, and centralized review of pathologic samples. The primary outcome measure is recurrence-free survival. Secondary outcome measures include:
- Safety
- Tolerability
- Progression-free survival
- Time to next intervention
Exploratory outcome measures include health-related quality of life and biomarker analyses. The study tests the hypothesis of 10% absolute improvement in recurrence free survival at 12 months in the treatment group with 90% power. The trial has received SUO-CTC and BCAN support, with 90+ clinical sites having been selected and screening/enrollment ongoing.
Presented by: Robert Svatek, MD, MSCI, UT-San Antonio, TX
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Society of Urologic Oncology (SUO) Annual Meeting, Dallas, TX, Tues, Dec 3 – Fri, Dec 6, 2024.
Related Content: PIVOT-006: Phase III Trial of Creto vs Surveillance in Intermediate-Risk Bladder Cancer - Mark Tyson


