(UroToday.com) The 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, between December 3 and December 6, 2024, was host to the Abstract/Posters Session. Dr. Ketty Bai discussed the utility of rescue Bacillus Calmette-Guerin therapy in treatment of BCG-unresponsive non-muscle-invasive bladder cancer.
Bacillus Calmette-Guérin (BCG) unresponsive non-muscle invasive bladder cancer (NMIBC) presents a substantial clinical challenge. Although several novel therapies have shown promise in treating BCG-unresponsive NMIBC, only a few have received FDA approval. The high costs and limited accessibility of these approved therapies hinder their widespread adoption, leaving patients with few treatment options. One such option is rechallenging with additional BCG, a practice that has largely fallen out of favor. However, contemporary analyses suggest that some patients may still benefit from this approach. Dr. Bai and her colleagues presented their experience with rescue BCG in patients with BCG-unresponsive disease, providing valuable insights into this potential treatment strategy.
The investigators conducted a retrospective review of an institutional database of patients diagnosed with NMIBC between 2003 and 2022 who were treated with BCG therapy according to the SWOG protocol. This protocol involves six weekly induction instillations of BCG, followed by three weekly maintenance instillations at months 3, 6, and every 6 months thereafter.
Patients meeting the criteria for BCG-unresponsive NMIBC were identified. A comprehensive chart review was conducted among these patients to determine the type of subsequent treatment they received after fulfilling the criteria for BCG-unresponsiveness. Only patients who received additional BCG therapy were included in the rescue BCG cohort and part of this analysis. Patients were divided into two cohorts:
- The rescue BCG cohort: these who received additional BCG therapy immediately after BCG-unresponsive designation. Those who received additional BCG plus interferon-alpha 2b
- No Rescue BCG cohort: Those who received alternative treatments such as intravesical chemotherapy immediately after BCG-unresponsive designation:
The primary outcomes of the study was progression-free survival (PFS), secondary outcomes were high-grade recurrence-free survival (HG-RFS) and overall survival. Calculated using the Kaplan-Meier method.
Dr. Bai noted that a total of 120 patients with BCG-unresponsive NMIBC were identified, of whom 66 were in the rescue BCG cohort and 54 in the No rescue BCG cohort. The median age of the rescue BCG cohort was 68 years, with most being male (70%). Over half (52%) of the patients treated with rescue BCG had T1 disease at initial diagnosis. The clinical profile of these patients underscores that this was a high-risk cohort. The median follow-up for the rescue BCG cohort was 79 months. (Table below)
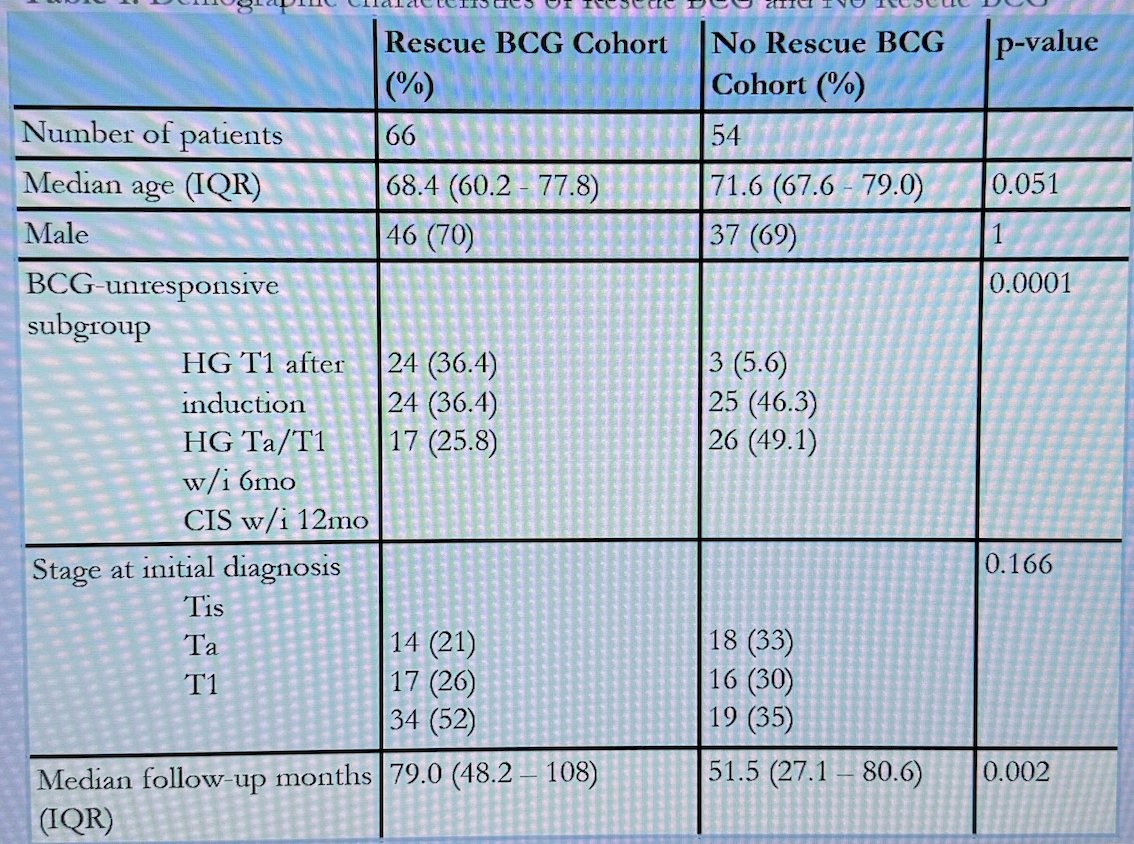
PFS for rescue BCG cohort at 1 year was 95% (89.9-100%), at 3 years 82.3% (72.8-92.9%) and at 5 years 77.7% (67.1-89.9%). The PFS in the No rescue chort was at 1 year 85.1%, 3 years 80.3% and at 5 years 72.9%. There were no significant differences in PFS between the two cohorts (p=0.38).
The therapies received in the No rescue BCG cohort were: intravesical chemotherapy (66.7%), surgery (18.5%), immunotherapy (3.7%).
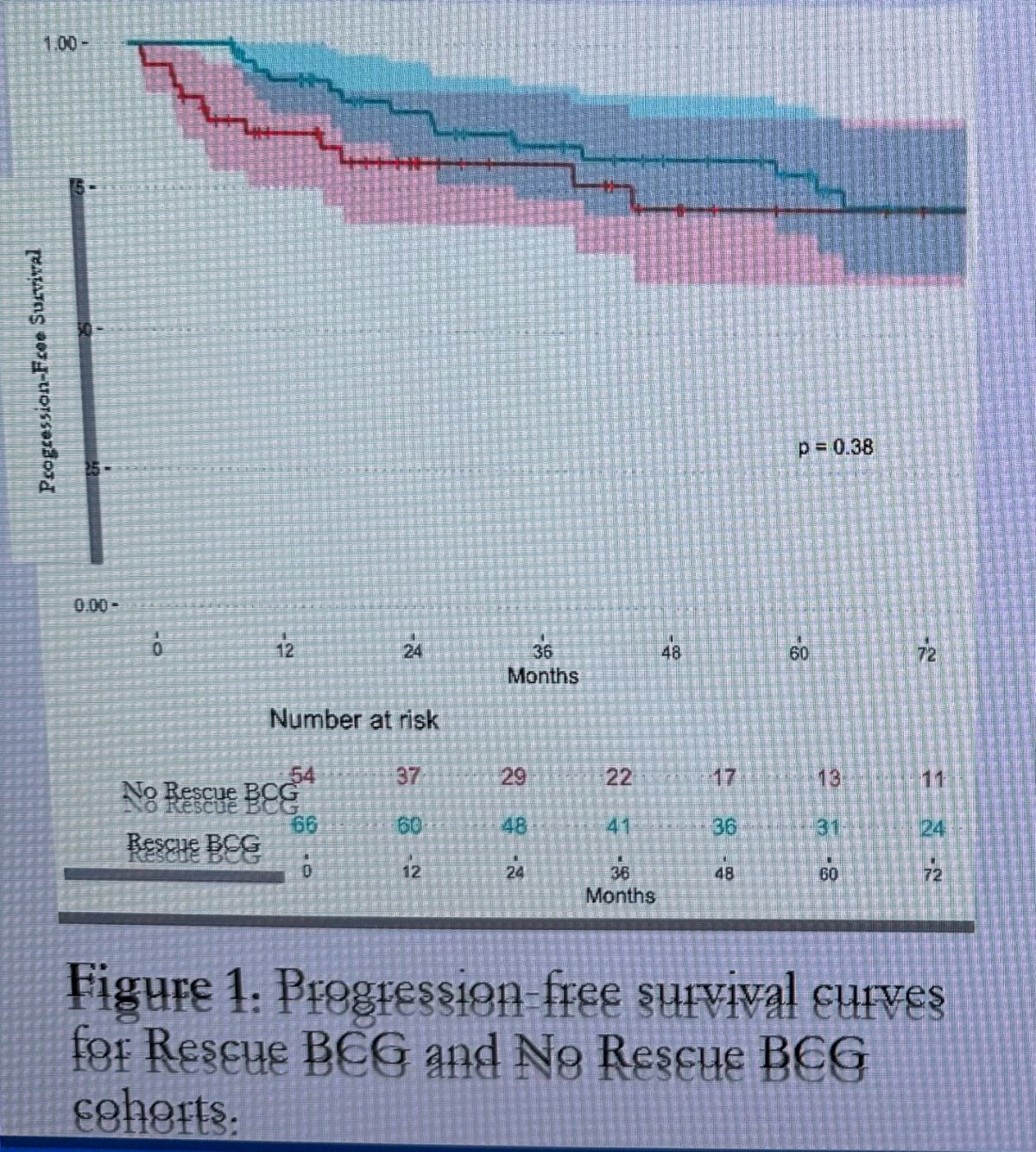
There were no significant differences (p=0.53) in HG-RFS between the rescue BCG and no rescue BCG cohorts.
To conclude their poster presentation, Ketty Bai gave the following key messages:
- The Rescue BCG cohort and the No Rescue BCG cohort had no significant differences in progression free survival, HG recurrence free survival, and overall survival.
- Using a Cox model to control for confounders, the investigators found that Rescue BCG was associated with a decreased risk of progression P= 0.048)
- Limitations of the study include a non-standardized strategy when selecting patients for Rescue BCG and the changes that occurred to the BCG unresponsive treatment landscape during the time span of the study (2003-2022)
Presented by: Ketty Bai, BS. Medical Student, Department of Urology, Columbia University, New. York, New York, USA
Written by: Julian Chavarriaga, MD – Staff Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, between the 3rd and 6th of December, 2024.


