(UroToday.com) The 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas between December 3 and December 6, 2024, hosted the Abstract/Posters Session. Dr. Hahn discussed the role of urine tumor DNA analysis in BCG-unresponsive non-muscle invasive bladder cancer (NMIBC) patients treated with durvalumab containing regimens in the HCRN GU16-243: ADAPT-BLADDER trial.
The presence of molecular residual disease (MRD) through the assessment of peripheral blood circulating tumor DNA (ctDNA) has significant clinical relevance in muscle-invasive (MIBC) and advanced bladder cancer patients.
Similarly, analyzing tumor DNA in patient urine specimens (utDNA) offers a promising approach to optimize clinical care and investigate tumor biology in non-muscle invasive bladder cancer (NMIBC) patients. The investigators explored the utility of mtDNA to characterize the genomic landscape and monitor MRD status in longitudinally collected urine samples from NMIBC patients treated with durvalumab-containing regimens in the previously reported HCRN GU16-243: ADAPT-BLADDER Trial.1
Baseline peripheral blood mononuclear cell for Predicine WES+ was collected pre-treatment, and longitudinal urine samples at baseline, 3-month, 6-month, tumor recurrence (if observed), and 24-month (if available) time points were collected from BCG-unresponsive NMIBC patients treated with durvalumab monotherapy, durvalumab plus BCG or durvalumab plus external beam radiation therapy (EBRT). The HCRN GU16-243: ADAPT-BLADDER trial schema is shown below:
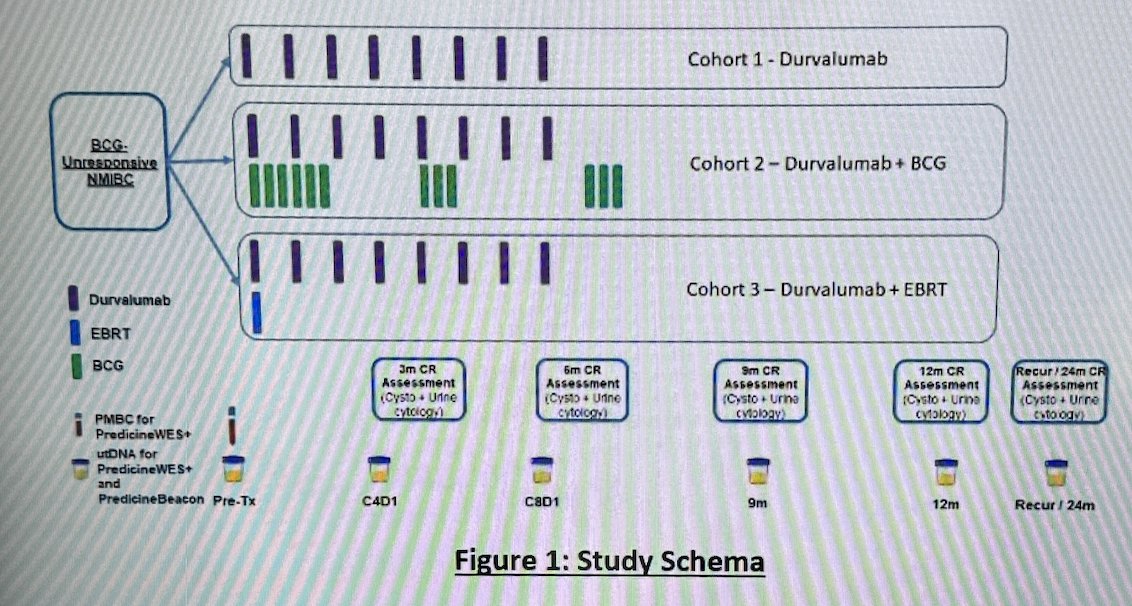
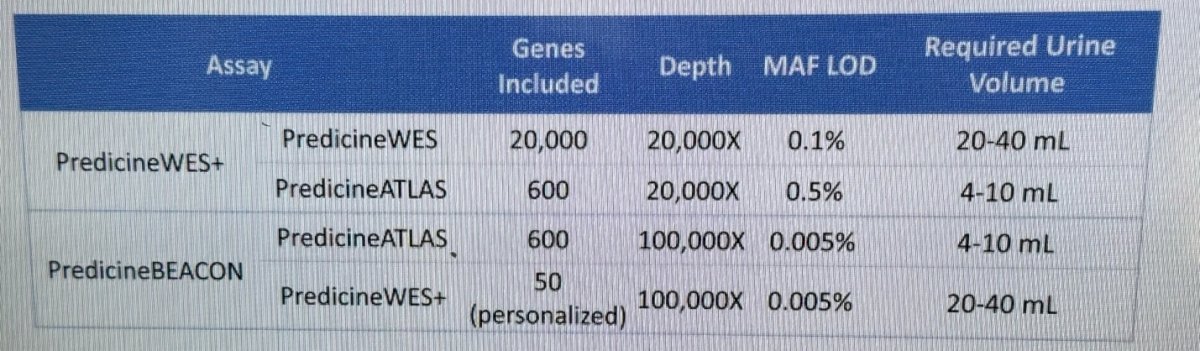
utDNA mutations, copy number aberrations, tumor mutational burden (TMB), and MRD status were assessed using the PredicineWES+ and PredicineBEACON MRD assays. The table below lists the genes included, the sequencing depth, the minimum allele frequency (MAF), and the required urine volume for each test:
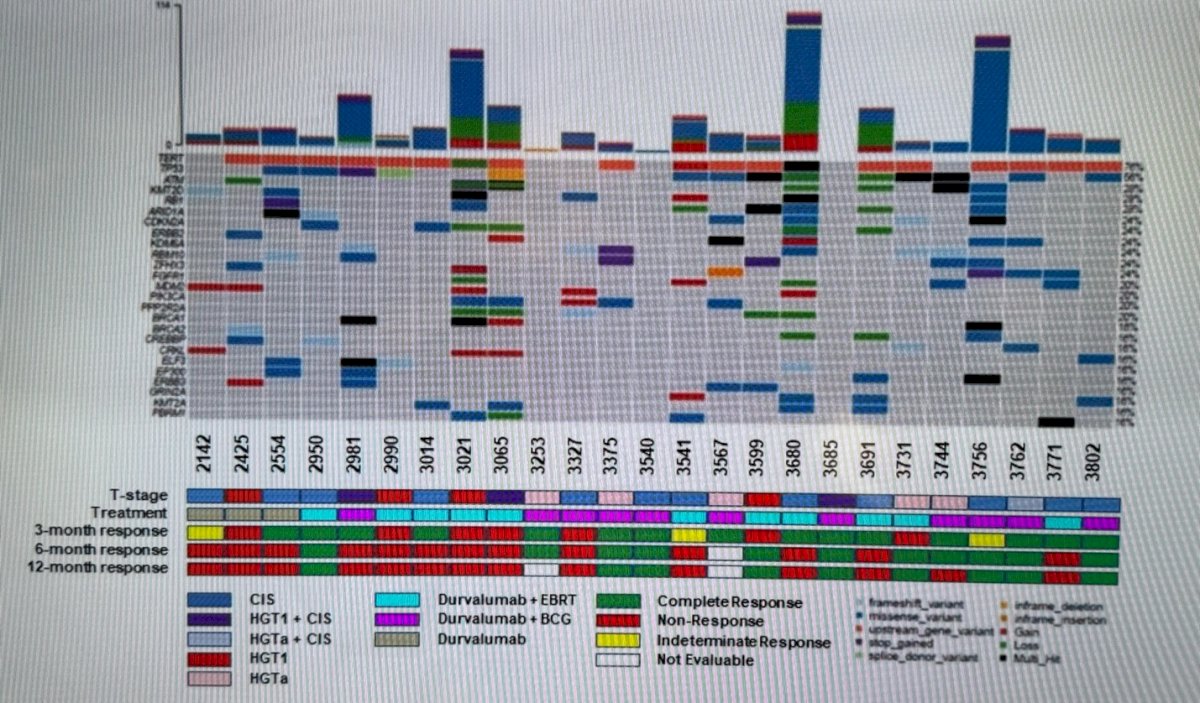
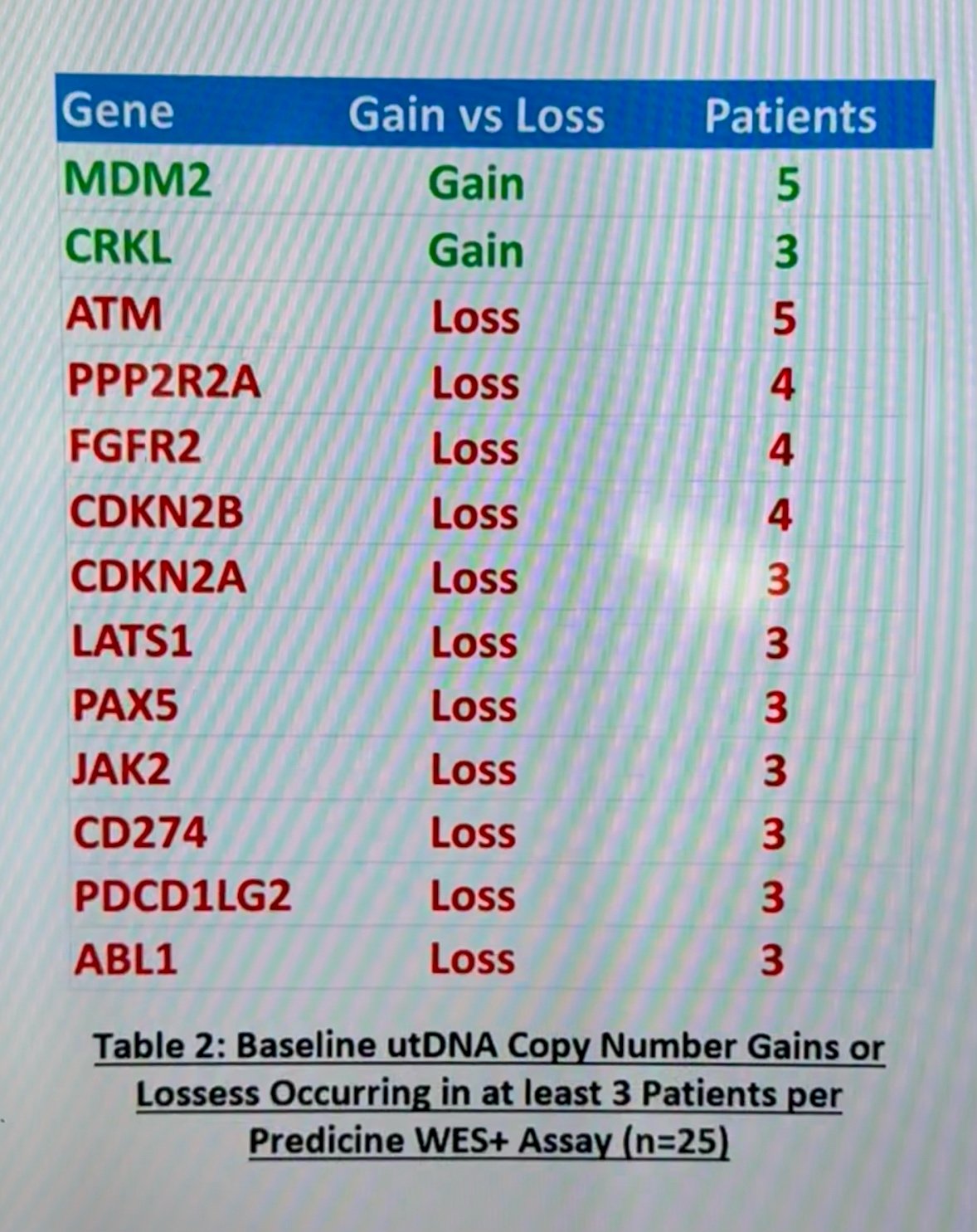
Samples from 25 patients were collected. Baseline utDNA was detectable in all (100%) patients with a median of 75 genomic alterations per sample, a median TMB of 1.4 muts/Mb (IQR 0.6 – 3.5), and a median tumor fraction (TF) of 21.0% (IQR 9.9 – 37.0). The baseline utDNA mutational landscape per PredicineWES+ assay can be appreciated in the figure below:
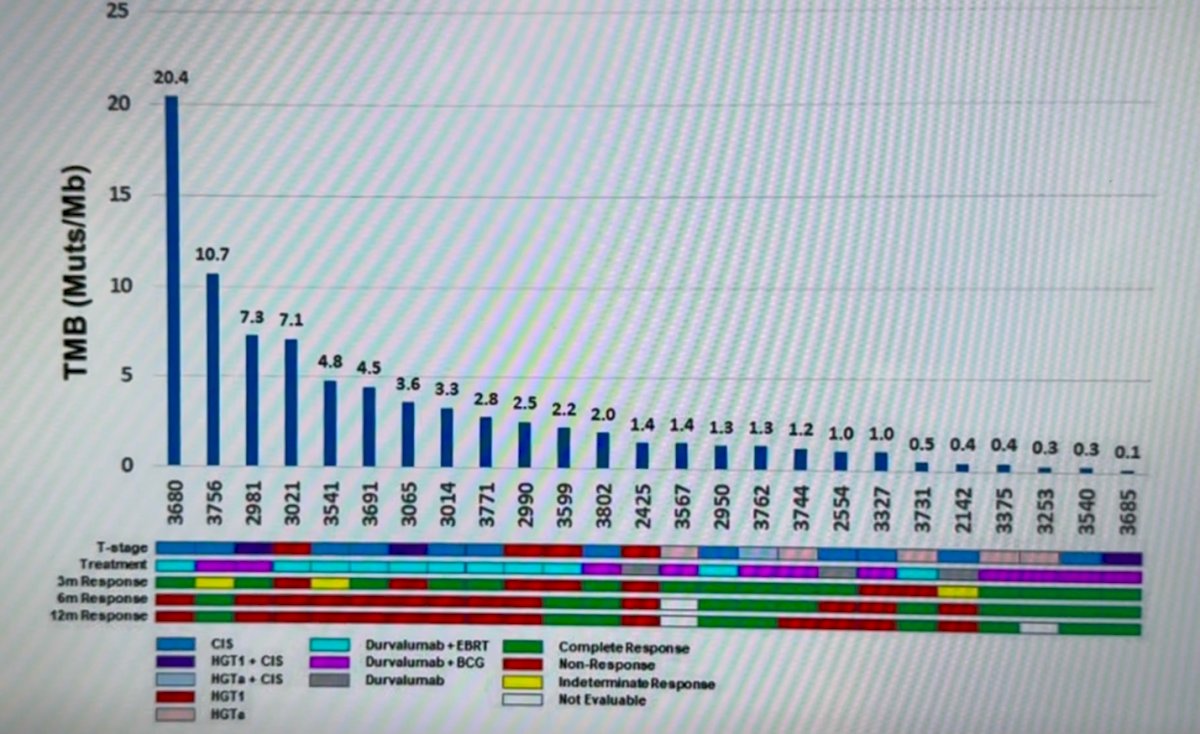
Notably, as reported earlier, the median TMB was 1.4 minutes/Mb; most patients had been treated with Durvalumab + EBRT or Durvalumab + BCG, and 15 (60%) had a complete response.
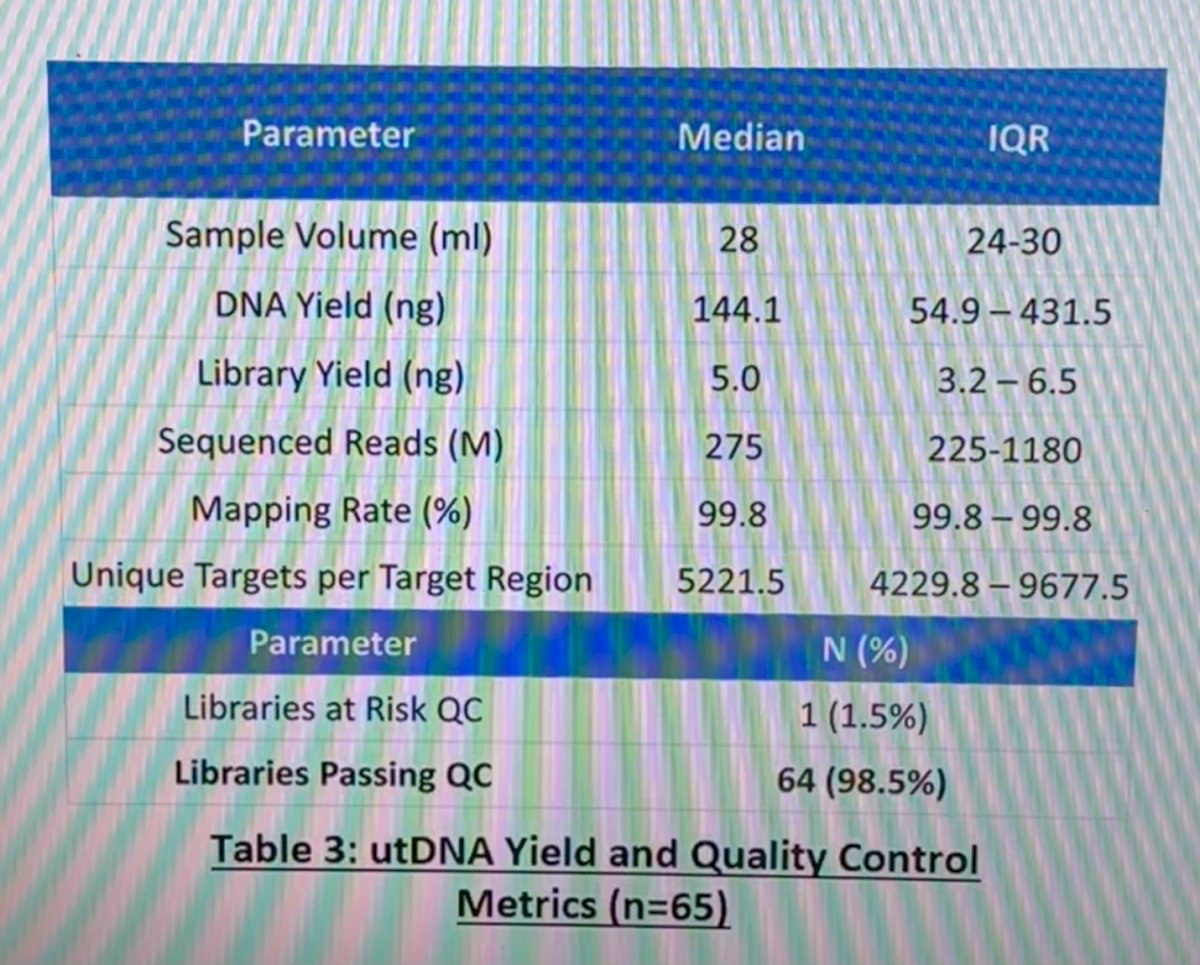
Regarding utDNA yield and quality control, the DNA yield was 144.1 ng, the mapping rate 99.8% and the libraries passing quality control 98.5%.
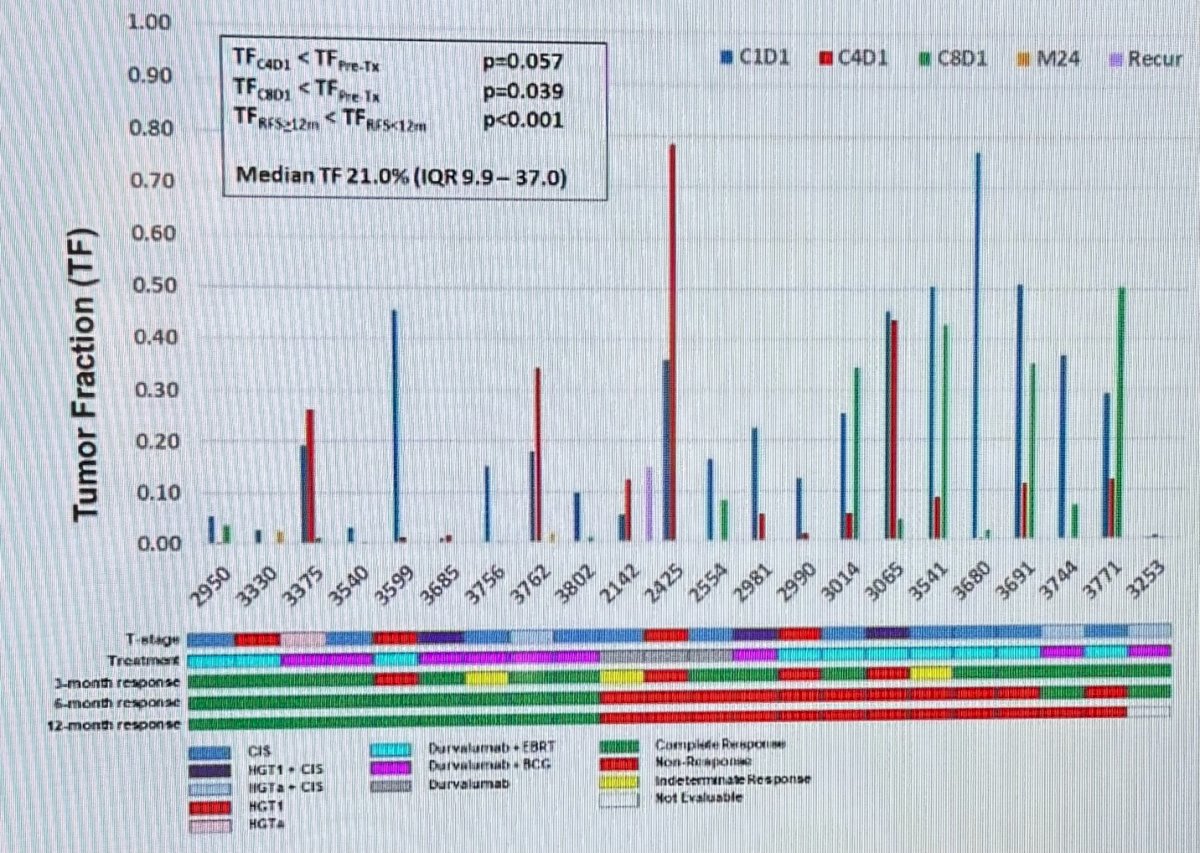
The median TF was 21% and in a multivariate GEE analysis, TF declined from baseline to cycle 4 (p=0.057), and to cycle 8 (p=0.039). TF was significantly lower in patients with RFS durations greater than than 12 months, p < 0.001.
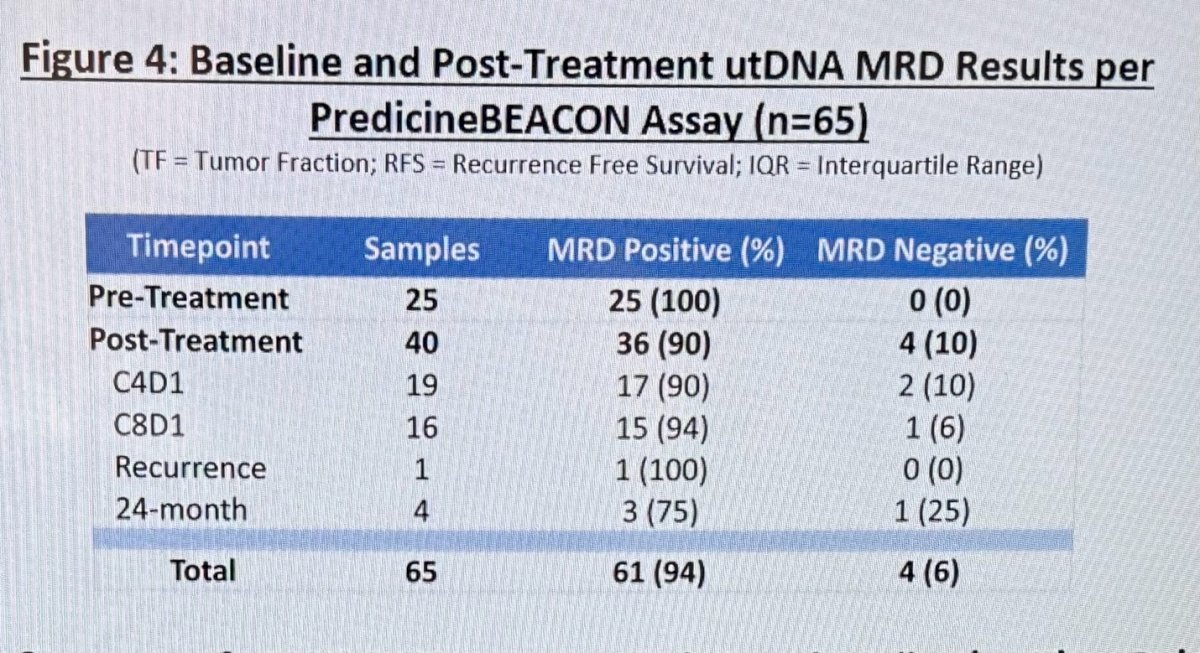
Following treatment, MRD remained detectable in 36 (90%) samples at 3 months, or C4D1 in 17/19 (90%) and at 6 months 15/16 (94%). Only one recurrence was documented, and it was MRD positive 1/1 (100%).
Dr Hahn concluded the poster presentation with the following messages:
- Characterization of utDNA genomic alterations and monitoring of utDNA MRD proved feasible using the PredicineWES+ and PredicineBEACON platforms in this initial investigation in BCG-unresponsive NMIBC patients treated with durvalumab-containing regimens in the HCRN GU16-243: ADAPT-BLADDER trial.
- Post-treatment utDNA MRD was detected in most (90%) samples.
- A post-treatment reduction in utDNA tumor fraction was associated with durable clinical benefit.
- Validation of the utility of utDNA MRD status as a novel biomarker of response in NMIBC patients is ongoing in more extensive randomized prospective trials.
Presented by: Noah M. Hahn, MD, Associate Professor of Oncology and Urology at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins in Baltimore, MD.
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas between the 3rd and 6th of December, 2024.
References:

