(UroToday.com) The 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX between December 3rd – 6th, 2024 was host to a bladder cancer poster session. Caretha Creasy presented a phase I dose-escalation study of UGN-301 (Zalifrelimab), both as monotherapy and in combination with other agents, in patients with recurrent non-muscle invasive bladder cancer (NMIBC).
Bladder cancer remains the sixth most common cancer in the USA (83,000 incident cases per year), with 75% being non-muscle invasive in nature. Approximately 15–30% of patients with high-grade NMIBC and over 50% of patients with intermediate-risk disease experience disease recurrence or progression, and, as such, new therapies in this disease space are sorely needed.
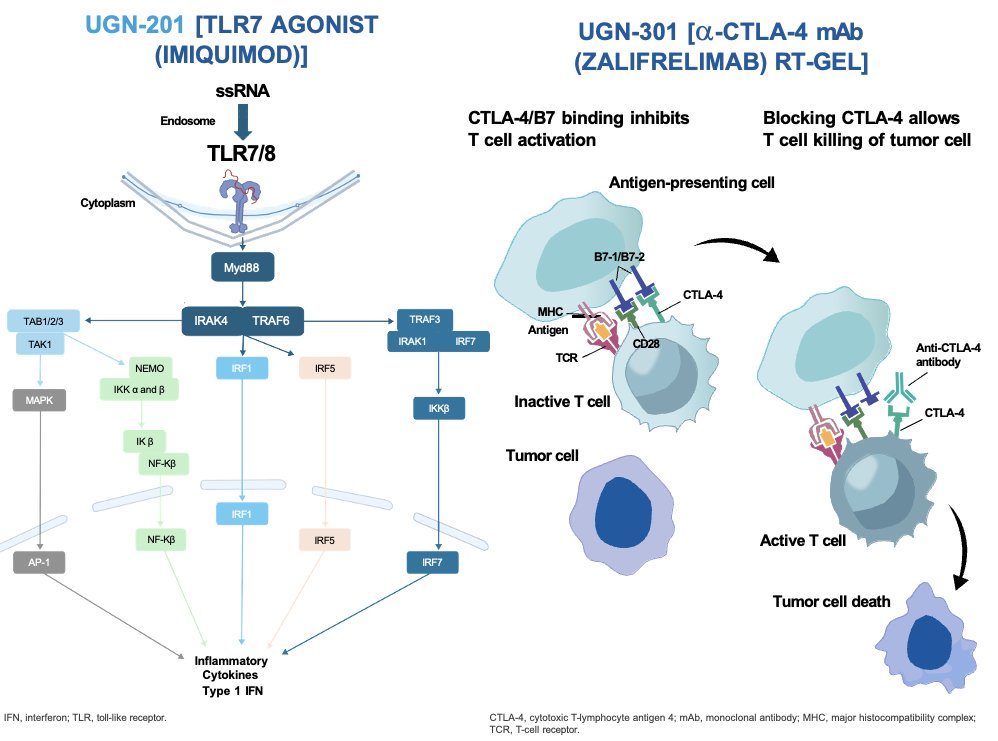
Zalifrelimab is an anti-cytotoxic T-lymphocyte antigen-4 (CTLA-4) monoclonal antibody that neutralizes the inhibitory effects of CTLA-4. UGN-301 is an intravesical formulation of zalifrelimab prepared with reverse thermal hydrogel (RTgel) and is expected to increase drug concentrations in the bladder without significant systemic exposure and without the toxicity associated with CTLA-4 blockade.
UGN-201 is a proprietary formulation of Imiquimod, a toll-like receptor 7 (TLR7) agonist. TLR7 dependent activation of the immune system causes a profound tumor-targeted cellular cytotoxic immune response and induces tumor cell apoptosis. It is delivered at room temperature as a liquid and is subsequently drained after an hour. Phase I and II trials have demonstrated clinical activity and increased urinary cytokines in NMIBC patients treated with imiquimod.
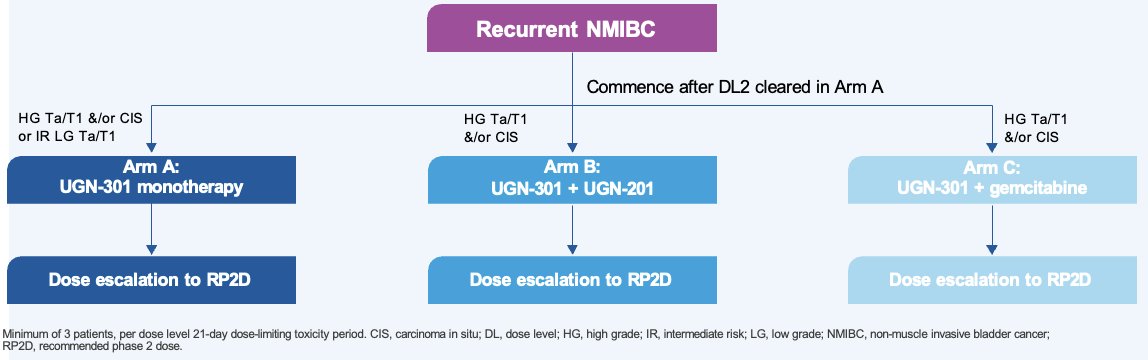
This is an ongoing multi-part phase I dose-escalation study, with up to 30 patients included per arm, to evaluate the safety and determine the recommended phase II dose (RP2D) of UGN-301 as monotherapy and in combination with other agents in patients with recurrent NMIBC. A Bayesian logistic regression model will be used to determine the biologically effective dose and maximum feasible dose of UGN-301.
The study design is illustrated below. Patients with recurrent NMIBC meeting specific eligibility criteria will be randomized to:
- Arm A: UGN-301 monotherapy
- HG Ta/T1 &/or CIS or intermediate risk LG Ta/T1
- Arm B: UGN-301 + UGN-201
- HG Ta/T1 &/or CIS
- Arm C: UGN-301 + gemcitabine
- HG Ta/T1 &/or CIS
The treatment and disease assessment schedule is illustrated below: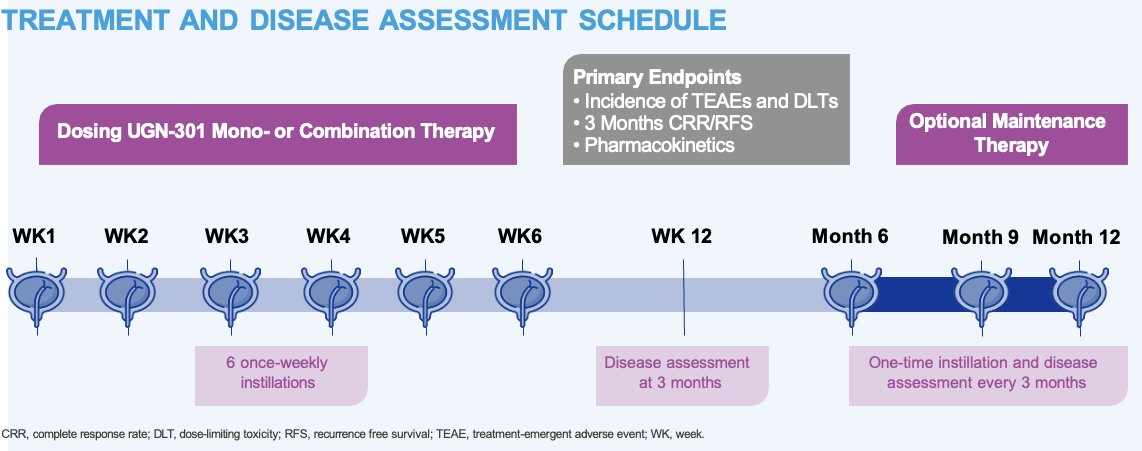
The key inclusion criteria are as follow:
- Patients with HG Ta/T1 disease and/or CIS must meet one of the following criteria:
- Have BCG-unresponsive disease and be unwilling or unfit to undergo radical cystectomy
- Have otherwise failed adequate BCG therapy (e.g. recurrence >6 months [papillary] or >12 months [CIS] after last BCG exposure)
- Are BCG intolerant, defined as the inability to tolerate at least one full induction course of BCG
- Have HG Ta disease with tumors ≤3 cm and failed at least one previous course of therapy (e.g. adjuvant intravesical chemotherapy)
- Have all papillary tumors visible by white light resected, and obvious areas of CIS fulgurated during screening or within 6 weeks before screening
The primary study objectives are to determine the:
- Biologically effective and maximum tolerated doses of UGN-301 as monotherapy and in combination with other agents in patients with recurrent NMIBC
- Endpoints: Incidence of treatment-emergent adverse events and dose-limiting toxicities
- Recommended phase II dose of UGN-301 as monotherapy and in combination with other agents, using the following endpoints:
- Incidence of treatment-emergent adverse events and dose-limiting toxicities
- Concentration of UGN-301 in blood and urine
- Complete response rate at 3 months, defined as the proportion of patients with CIS who achieved a complete response at the Week 12 (3 month) visit as determined by cystoscopy, for-cause biopsy, and urine cytology
- Recurrence-free survival rate at 3 months in patients with Ta/T1 disease, defined as the proportion of patients who are recurrence-free at the Week 12 (3 month) visit as determined by cystoscopy, for-cause biopsy, and urine cytology
The secondary and exploratory endpoints are as follows: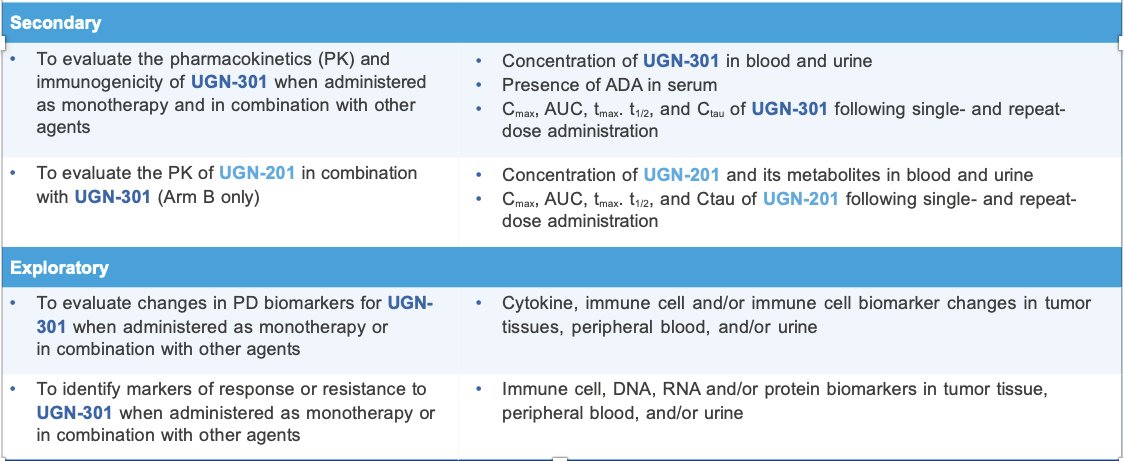
Study recruitment into this trial is currently ongoing.
Presented by: Caretha L. Creasy, PhD, Vice President, Early Development at UroGen Pharma, Princeton, New Jersey
Written by: Rashid K. Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX between December 3rd and 6th, 2024


