(UroToday.com) The 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX between December 3rd – 6th, 2024 was host to a bladder cancer poster session. Dr. Shilpa Gupta presented the study design of Cohort C of the phase II KEYNOTE-057 trial comparing the co-formulations of Vibostolimab/pembrolizumab and Favezelimab/pembrolizumab for bacillus Calmette-Guérin (BCG)-unresponsive, high-risk, non-muscle invasive bladder cancer (NMIBC).
Pembrolizumab, a PD-1 inhibitor, was shown to have anti-tumor activity in cohort A of the open-label phase II KEYNOTE-057 trial (NCT02625961), leading to the US Food and Drug Administration (FDA) approval of pembrolizumab for the treatment of patients with BCG–unresponsive high-risk (HR) NMIBC with carcinoma in situ (CIS) with or without papillary tumors who are ineligible for or have elected not to undergo radical cystectomy.1,2
Novel combinations that can further improve the efficacy of pembrolizumab are urgently needed. The immune checkpoints T-cell immunoglobulin and ITIM domain (TIGIT) and lymphocyte activation gene 3 (LAG-3) have been shown to contribute to treatment resistance in many cancers and have been expressed in bladder cancer. Compared with healthy donors, increased peripheral blood total NK cells and higher TIGIT expression on NK, CD4+, and CD8+ T cells have been observed in patients with NMIBC.3 Stromal expression of LAG-3 has been shown to correlate with poor prognosis in metastatic urothelial cancer and expression in immune cell subsets has been found to correlate with PD-1 expression.4
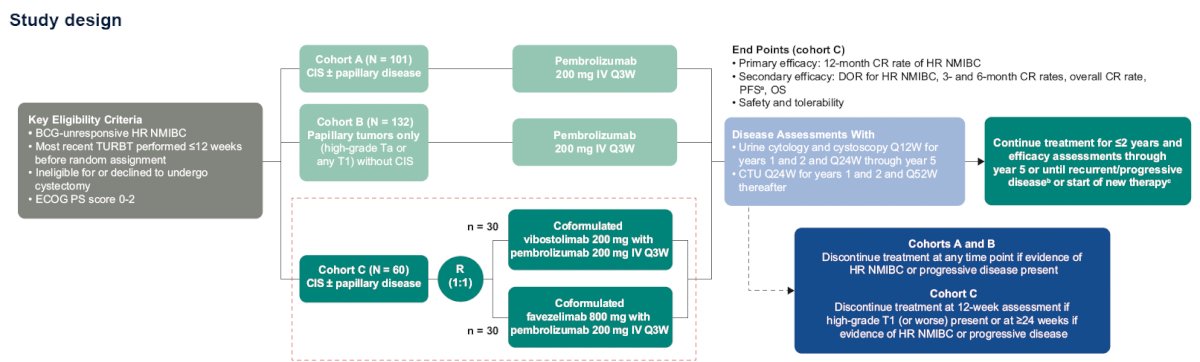
Because inhibition of TIGIT and LAG-3 may enhance the efficacy of pembrolizumab, cohort C of KEYNOTE-057 will evaluate the efficacy and safety of coformulations (receiving both treatments as a single infusion) of the TIGIT inhibitor vibostolimab with pembrolizumab and of the LAG-3 inhibitor favezelimab with pembrolizumab in patients with high-risk, BCG-unresponsive NMIBC with CIS +/- papillary tumors
The study design is illustrated below. In Cohort C, 60 patients with BCG-unresponsive CIS +/- papillary disease will undergo 1:1 randomization to:
- Co-formulated vibostolimab 200 mg + pembrolizumab 200 mg IV every 3 weeks
- Co-formulated favezelimab 800 mg+ pembrolizumab 200 mg IV every 3 weeks
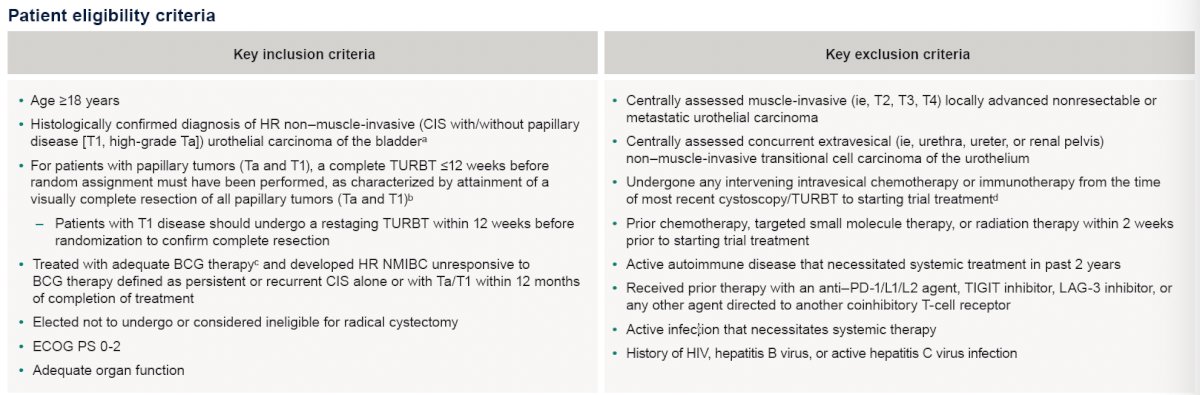
The patient eligibility criteria are summarized below:
The primary study objective is to evaluate the antitumor activity of the coformulations using the endpoint of 12-month complete response rate of high-risk NMIBC, as determined by cystoscopy, cytology, biopsy, and radiologic imaging by central pathology and radiology review.
The secondary study endpoints are as follows:
- Duration of response of high-risk NMIBC in responders
- Complete response rates at 3 and 6 months
- Overall complete response rate
- Progression-free survival to worsening of grade, stage, or death
- Progression-free survival to muscle-invasive or metastatic disease or death
- Overall survival
- Safety
The study assessments and follow-ups are summarized below: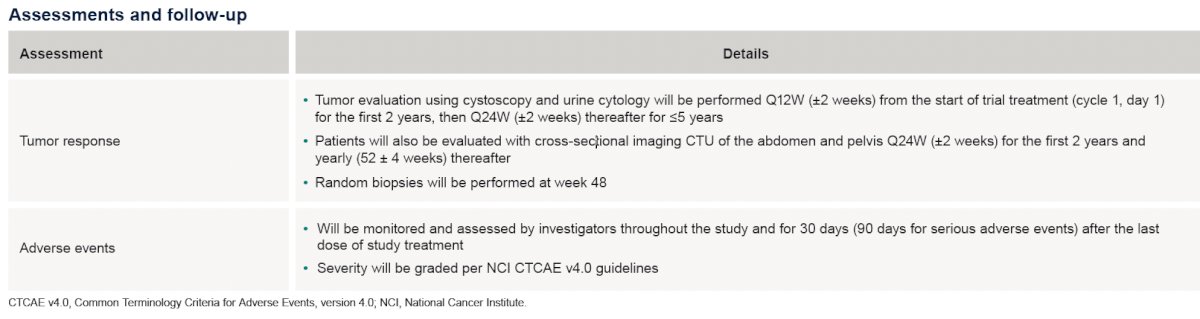
KEYNOTE-057 cohort C is enrolling patients at sites in Asia, Australia, Europe, North America, and South America.
Presented by: Shilpa Gupta, MD, Professor, Director of the Genitourinary Medical Oncology at Taussig Cancer Institute and Co-Leader of the Genitourinary Oncology Program at Cleveland Clinic, Cleveland, OH
Written by: Rashid K. Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX between December 3rd and 6th, 2024
- Balar AV, Kamat AM, Kulkarni GS, et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): An open-label, single-arm, multicenter, phase 2 study. Lancet Oncol. 2021; 22(7):919-90.
- FDA approves pembrolizumab for BCG-unresponsive, high-risk non-muscle invasive bladder cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-bcg-unresponsive-high-risk-non-muscle-invasive-bladder-cancer. Accessed on December 6, 2024.
- Audenet F, Farkas AM, Anastos H, et al. Immune phenotype of peripheral blood mononuclear cells in patients with high-risk non-muscle invasive bladder cancer. World J Urol. 2018; 36(11):1741-8.
- Zeng H, Zhou Q, Wang Z, et al. Stromal LAG-3+ cells infiltration defines poor prognosis subtype muscle-invasive bladder cancer with immunoevasive contexture. J Immunother Cancer. 2020; 8(1):e000651.


