(UroToday.com) The 2024 SUO annual meeting included a urothelial carcinoma session, featuring a presentation by Dr. Vikram Narayan discussing the incidence and pathologic outcomes of cystectomy in patients with BCG-unresponsive non muscle invasive bladder cancer (NMIBC) with CIS following treatment with nadofaragene firadenovec. Patients with BCG–unresponsive NMIBC are at significant risk for recurrence and progression.
Although cystectomy is a recommended definitive treatment, it is associated with morbidity and mortality. Nadofaragene firadenovec-vncg is a non-replicating recombinant adenovirus vector-based gene therapy that delivers human interferon alpha-2b cDNA into the bladder urothelium. In the CS-003 phase 3 study, 55/103 participants (53.4%) with CIS with or without papillary tumors (± Ta/T1) achieved a complete response by 3 months.1 Furthermore, 25 of 55 patients (45.5%) with an initial 3 month complete response maintained response through 12 months. At the SUO 2024 annual meeting, cystectomy-free survival and pathologic outcomes at cystectomy were evaluated among participants with BCG-unresponsive NMIBC with CIS ± Ta/T1 treated with nadofaragene firadenovec, stratified by complete response status at 3 months. The objective of this analysis was to test the hypothesis that nadofaragene firadenovec would enable many participants to avoid cystectomy without compromising the window of cure for those who did not achieve or maintain a complete response.
An open-label, multicenter, phase 3 study enrolled participants with BCG-unresponsive NMIBC to receive a single dose of nadofaragene firadenovec, with repeat dosing every 3 months in the absence of high-grade recurrence: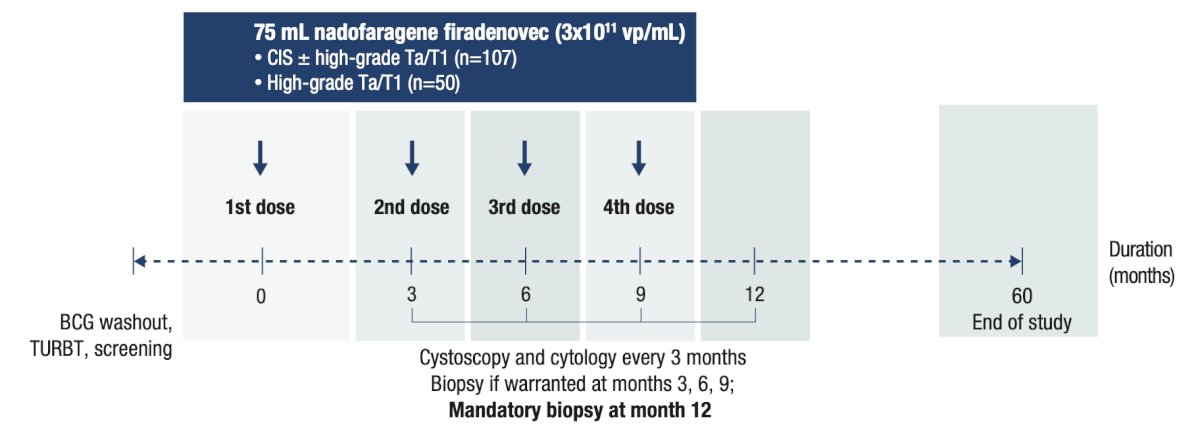 Incidence of cystectomy and cystectomy-free survival (calculated from the first dose of nadofaragene firadenovec to the first date of cystectomy or death due to any cause) were assessed through 5 years of follow-up. Cystectomy pathology reports were assessed through the 36-month data cutoff (the 36-month visit of the last enrolled participant) to evaluate stage and progression. Results are presented for the overall CIS ± Ta/T1 population and stratified by complete response status at 3 months. The median follow-up time was 48.9 months among all treated participants with CIS ± Ta/T1 (n = 107):
Incidence of cystectomy and cystectomy-free survival (calculated from the first dose of nadofaragene firadenovec to the first date of cystectomy or death due to any cause) were assessed through 5 years of follow-up. Cystectomy pathology reports were assessed through the 36-month data cutoff (the 36-month visit of the last enrolled participant) to evaluate stage and progression. Results are presented for the overall CIS ± Ta/T1 population and stratified by complete response status at 3 months. The median follow-up time was 48.9 months among all treated participants with CIS ± Ta/T1 (n = 107):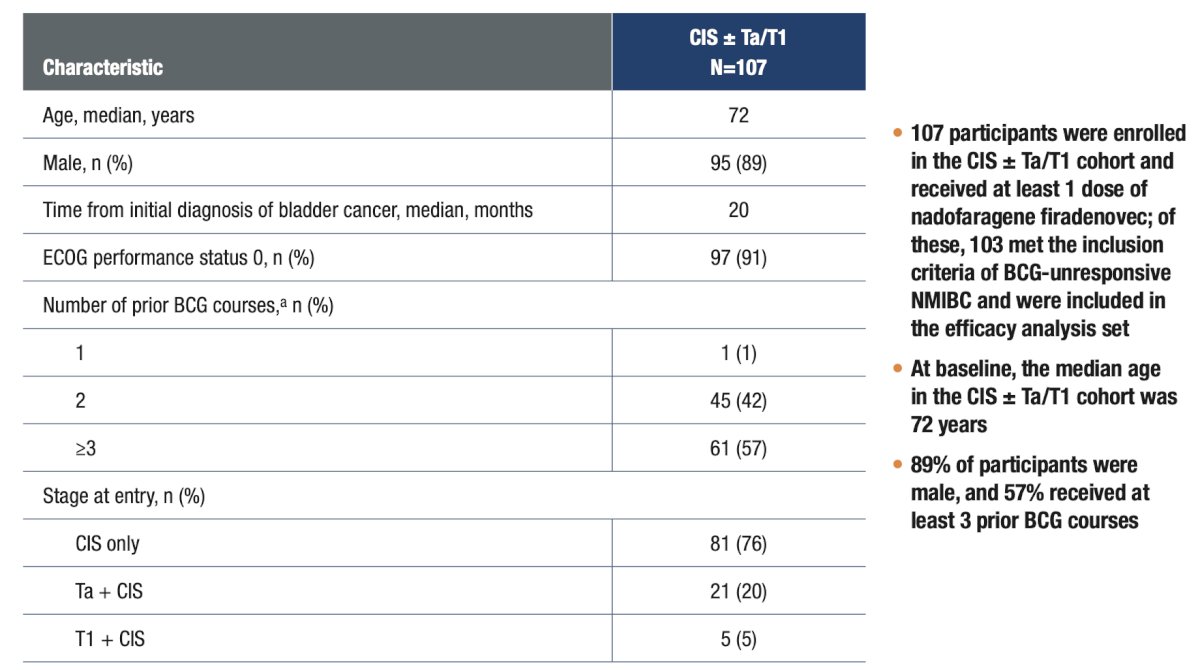
Among participants included in efficacy analyses (n = 103), 44 (42.7%) underwent cystectomy, including 15 (14.6%) who achieved a complete response at 3 months and 29 (28.2%) who did not. Of the 37 participants who underwent cystectomy with pathology data available, 6 had muscle invasive bladder cancer at cystectomy, of whom 3 had muscle invasive bladder cancer at transurethral resection before cystectomy and 3 underwent cystectomy for clinical NMIBC. Three participants were pT0 at cystectomy. The Kaplan–Meier estimated median duration of cystectomy-free survival was 47.9 months, 63.9 months, and 11.3 months in all participants with CIS ± Ta/T1, participants with a complete response, and participants without a complete response, respectively: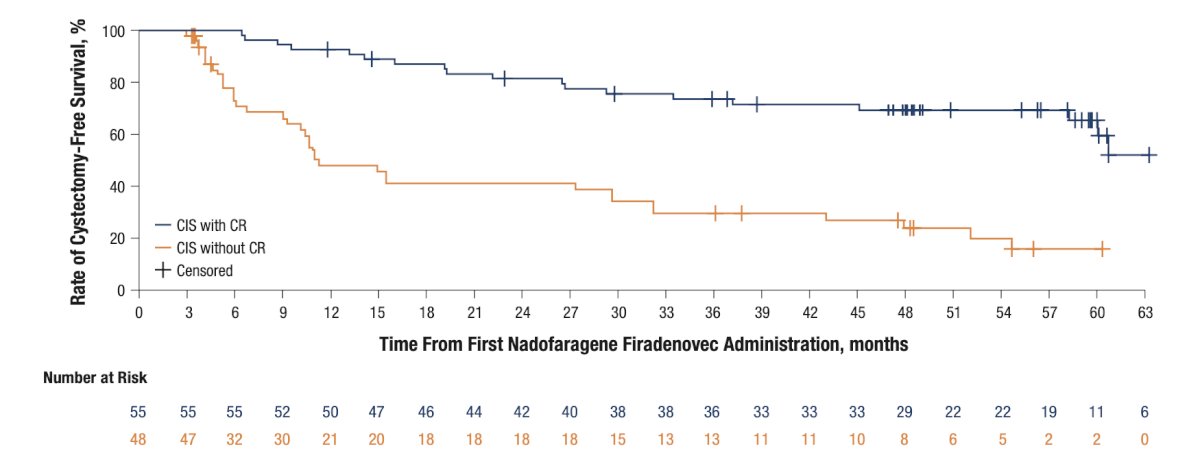
The Kaplan–Meier probability of cystectomy-free survival for at least 60 months was 43.2%, 65.5%, and 16.0% in all participants with CIS ± Ta/T1, participants with a complete response, and participants without a complete response, respectively: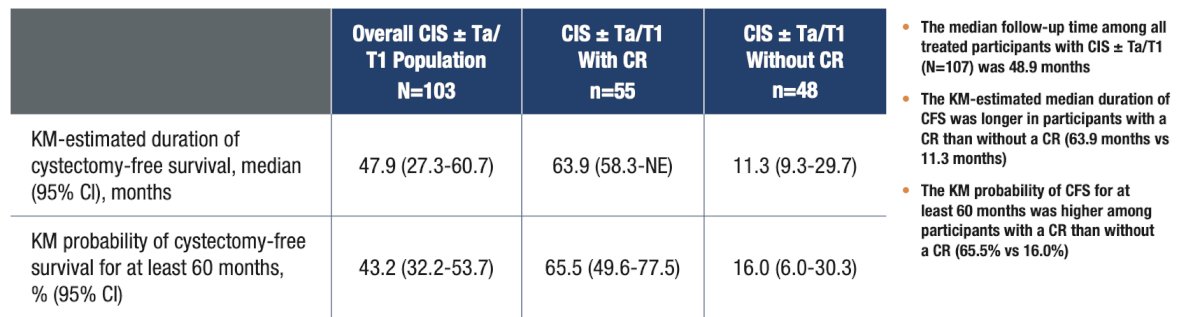
Time from recurrence to cystectomy, death, or last evaluable time point in patients with CIS ± Ta/T1 (A) with (n = 41) and (B) without (n = 48) complete response at 3 months in the efficacy analysis is as follows: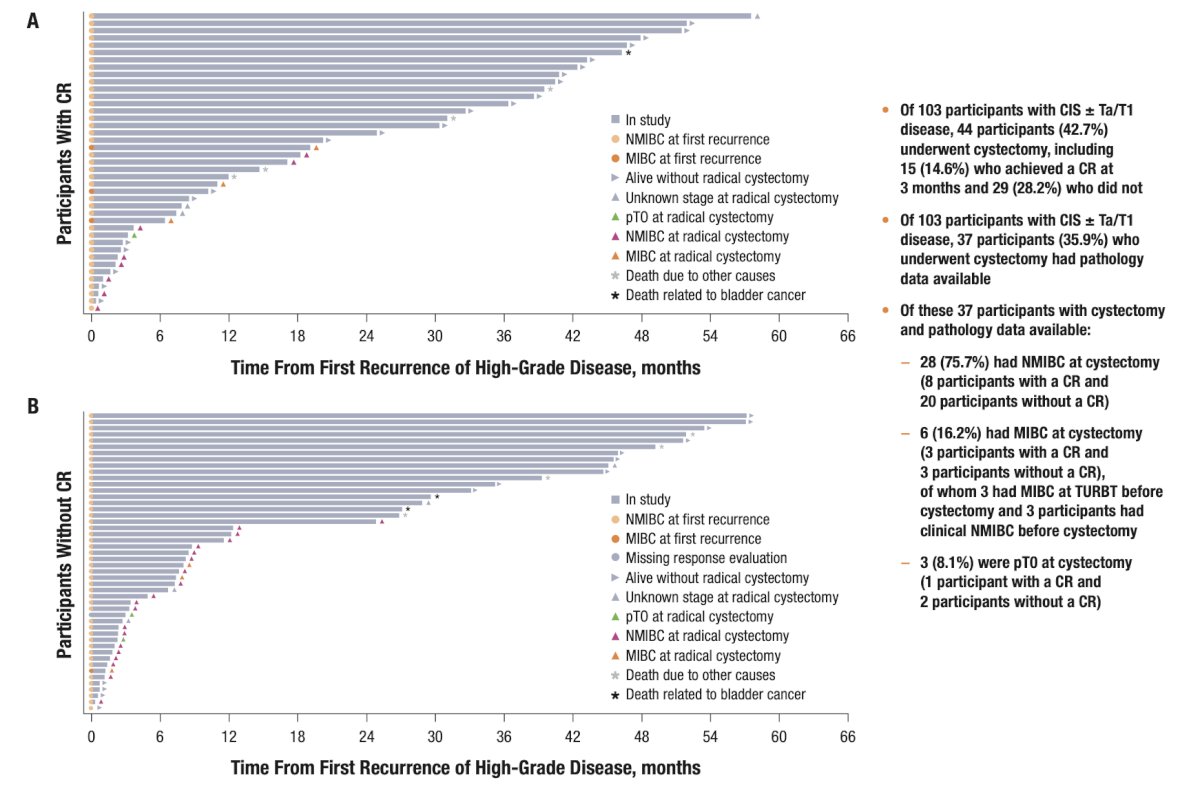
Dr. Narayan concluded his presentation discussing the incidence and pathologic outcomes of cystectomy in patients with BCG-unresponsive NMIBC with CIS following treatment with nadofaragene firadenovec with the following take home messages:
- Participants with BCG-unresponsive NMIBC with CIS ± Ta/T1who had an initial complete response at 3 months following nadofaragene firadenovec treatment demonstrated a ~66% Kaplan-Meier estimated probability to be alive and cystectomy-free at 60 months
- The rate of upstaging at the time of cystectomy was consistent with historical reports on immediate cystectomy for NMIBC, indicating nadofaragene firadenovec is a safe quarterly administered bladder-sparing option that does not sacrifice the window of cure for patients with BCG-unresponsive NMIBC
Presented by: Vikram M. Narayan, MD, Emory University, Atlanta, GA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Society of Urologic Oncology (SUO) Annual Meeting, Dallas, TX, Tues, Dec 3 – Fri, Dec 6, 2024.
References:


