(UroToday.com) The 2024 SUO annual meeting included a urothelial carcinoma session, featuring a presentation by Dr. Vignesh Packiam discussing an artificial intelligence powered model to predict response to intravesical BCG versus gemcitabine-docetaxel for high risk non muscle invasive bladder cancer (NMIBC). Intravesical BCG is first-line adjuvant therapy for high risk NMIBC given the ability to reduce recurrence and progression of disease.
EA-8212 (BRIDGE) is evaluating BCG versus gemcitabine and docetaxel in NMIBC, though existing analyses in treatment naïve disease suggest sequential intravesical gemcitabine and docetaxel has increasingly shown promising results as therapy in the BCG-naive setting. Biomarkers predicting response to BCG versus gemcitabine and docetaxel could have an immediate impact on disease management. Recently, Dr. Packiam and colleagues demonstrated a computational histology artificial intelligence (CHAI) assay that predicts response to BCG using digital images of H&E pathology slides from pre-treatment TURBT specimens:
Presence of the biomarker conferred an increased risk of recurrence, progression, and BCG unresponsive disease. At SUO 2024, Dr. Packiam and colleagues aimed to assess if this biomarker was predictive of response to BCG versus gemcitabine and docetaxel.
Pre-treatment TURBT-derived digital whole slide images and clinical data were obtained for patients with BCG-naïve AUA high risk NMIBC diagnosed between 2011-2021 and treated with BCG or gemcitabine and docetaxel. Whole slide images were analyzed using the CHAI assay incorporating histologic features previously shown to be predictive of development of BCG unresponsive disease. Those with “biomarker present” were previously shown to have a shorter time to BCG unresponsive disease than those with “biomarker absent”. High-grade recurrence-free survival between BCG and gemcitabine and docetaxel were compared in the pre-defined biomarker present and absent groups. A test for statistical interaction between the biomarker and treatment type was assessed via Cox proportional hazards regression and likelihood ratio tests.
There were 273 patients with high risk NMIBC, of whom 59% received BCG and 41% received gemcitabine and docetaxel. The median follow-up was 35 months. Demographic and tumor characteristics were similar between patients receiving either treatment and in either CHAI biomarker category. High grade recurrence free survival at 24 months was worse in BCG-treated versus gemcitabine and docetaxel treated patients (70% versus 84%, p < 0.02):
There were 80 (29%) patients that were CHAI biomarker present: 45 (56%) received BCG, and 35 (44%) received gemcitabine and docetaxel. In BCG treated patients, CHAI biomarker present cases had inferior high grade recurrence free survival compared to CHAI biomarker absent cases (HR 2.0, 95% CI 1.1 – 3.6, p < 0.02):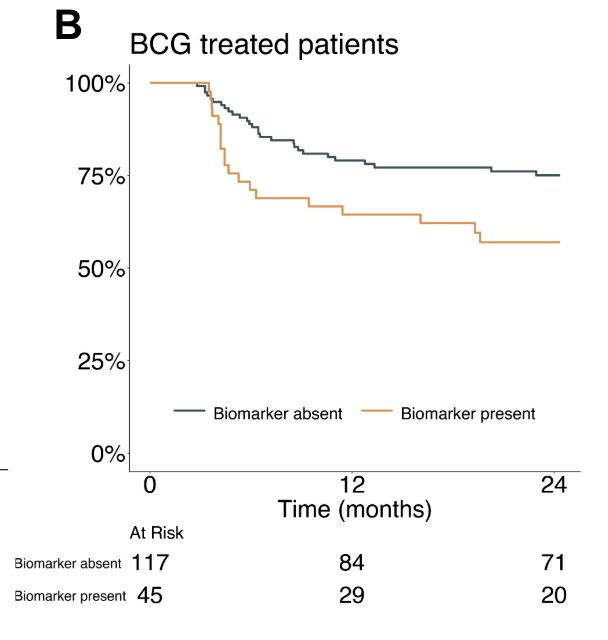
Among CHAI biomarker present cases, gemcitabine and docetaxel treated patients had superior high grade recurrence free survival compared to BCG treated patients (HR 8.2, 95% CI 1.9 – 35.4, p = 0.005):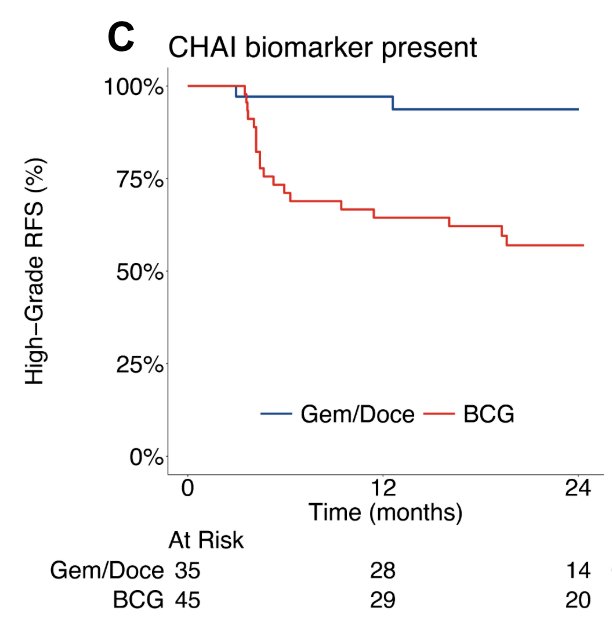
The 24 month high grade recurrence free survival in gemcitabine and docetaxel treated cases was 94% versus 57% in those receiving BCG (p < 0.001). Among CHAI biomarker negative cases, there was no significant difference in high grade recurrence free survival between gemcitabine and docetaxel versus BCG treated cases (HR 1.2, 95% CI 0.6 – 2.3, p = 0.50):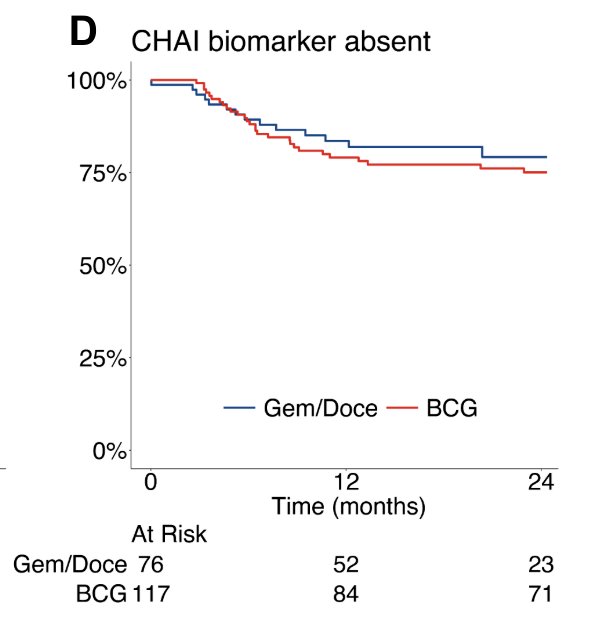
The likelihood ratio test for the biomarker treatment interaction term was statistically significant (p = 0.006) indicating the CHAI biomarker is predictive: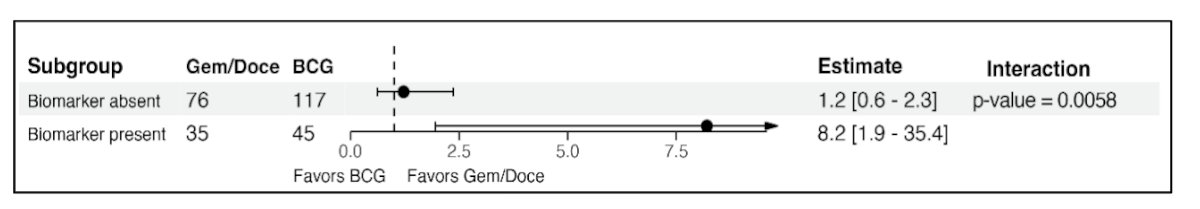
Dr. Packiam concluded his presentation discussing an artificial intelligence powered model to predict response to intravesical BCG versus gemcitabine-docetaxel for high risk NMIBC with the following take home messages:
- In the treatment-naïve setting for high grade NMIBC, BCG is the “gold standard”. However, the ongoing shortage limits patient access to BCG, and there is an unmet need for predictors of response to therapies
- Intravesical gemcitabine and docetaxel has increasing evidence supporting its viability as a BCG alternative
- This study is the first to evaluate an artificial intelligence based histologic biomarker to predict clinical response to different intravesical therapies in high grade NMIBC
- The CHAI biomarker is statistically predictive of treatment benefit. CHAI biomarker presence conferred superior high grade recurrence free survival in patients treated with gemcitabine and docetaxel as compared to BCG
- The CHAI biomarker enables more personalized medicine in NMIBC, predicting clinical response to BCG versus gemcitabine and docetaxel, thus sparing unnecessary delays and toxicity in patients unlikely to benefit
Presented by: Vignesh Packiam, MD, Rutgers Cancer Institute of New Jersey, New Brunswick, NJ
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Society of Urologic Oncology (SUO) Annual Meeting, Dallas, TX, Tues, Dec 3 – Fri, Dec 6, 2024.


