(UroToday.com) The 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, between December 3 and December 6, 2024, was host to the Abstract/Posters Session. Dr. Gabrielle Yankelevich discussed their multicenter experience with first-line intravesical gemcitabine-docetaxel versus Bacillus Calmette-Guerin for high-risk non-muscle invasive bladder cancer (NMIBC).
There is a global shortage of BCG due to the growing demand for this product worldwide. As a result, many patients are unable to complete the recommended course of BCG treatment, leading some to switch to alternative agents. Recurrence rates within two years after BCG therapy range from 30-50%.1,2 Consequently, the use of intravesical chemotherapy for both primary and salvage treatments after BCG failure is increasing. This highlights the ongoing challenges in managing non-muscle invasive bladder cancer amid BCG supply issues. The investigators aimed to determine if Gemcitabine/Docetaxel (Gem/Doce) could be associated with similar oncologic efficacy as BCG as the first-line treatment for high-risk non-muscle invasive bladder cancer (HR-NMIBC).
This was a multi-center, retrospective cohort study analyzing 136 patients with HR-NMIBC treated between August 2020 and August 2023. Patients were included in the study if they required Gem-Doce or BCG induction and had the option for maintenance therapy. Those with a history of upper tract urothelial carcinoma were excluded from the study.
The primary endpoint of the study was high-grade (HG) recurrence free survival assessed during surveillance cystoscopy and urine cytology performed every three months. The oncological efficacy of Gem-Doce vs. BCG was evaluated at 12 and 24 months.
A total of 55 patients received Gem/Doce and 81 patients received BCG. The baseline characteristics were well balanced between both groups; however, patients in the Gem/Doce group were older (73 vs. 71 years) and more likely to have multifocal disease (62% vs. 34%) compared to the BCG group. The rest of the baseline characteristics are shown in the table below:
The median follow-up was 21 months, and during this period, 30 high-grade recurrences were documented: 12 in the Gem/Doce group and 18 in the BCG group. The 24-month recurrence-free survival (RFS) rates were 74% for the Gem/Doce group and 81% for the BCG group. Intravesical Gem-Doce did not show a significant difference in recurrence-free survival compared to BCG (p=0.53), as illustrated in the Kaplan-Meier graphic below: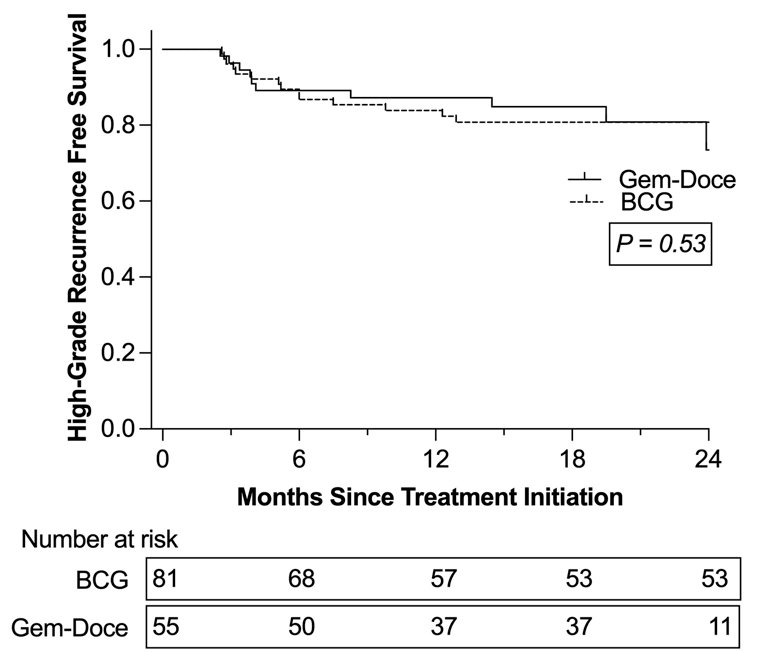
Dr. Yankelevich concluded her poster presentation by saying that:
- First-line intravesical Gem-Doce for HR-NMIBC demonstrates comparable oncologic efficacy to BCG.
- Future studies should focus on assessing the tolerability of Gem-Doce, long-term follow-up regarding adverse events, and evaluating cystectomy-free survival as a key endpoint.
Presented by: Gabrielle Yankelevich, MD, Urology PGY4 at the Ralph H. Johnson Veterans Affairs Medical Center, Charleston, South Carolina.
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, between the 3rd and 6th of December, 2024.
References:
- Kates M, Chu X, Hahn N, Pietzak E, Smith A, Shevrin DH, Crispen P, Williams SB, Daneshmand S, Packiam VT, Porten S, Westerman ME, Wagner LI, Carducci M. Background and Update for ECOG-ACRIN EA8212: A Randomized Phase 3 Trial of Intravesical Bacillus Calmette-Guérin (BCG) Versus Intravesical Docetaxel and Gemcitabine Treatment in BCG-naïve High-grade Non-muscle-invasive Bladder Cancer (BRIDGE). Eur Urol Focus. 2023 Jul;9(4):561-563. doi: 10.1016/j.euf.2023.06.006.
- McElree IM, Steinberg RL, Martin AC, Richards J, Mott SL, Gellhaus PT, et al. Sequential Intravesical Gemcitabine and Docetaxel for bacillus Calmette-Guérin-Naïve High-Risk Nonmuscle-Invasive Bladder Cancer. Journal of Urology. 2022. 208(3):589–99. doi:10.1097/JU.0000000000002740


