(UroToday.com) The 2024 SUO annual meeting included a urothelial carcinoma session, featuring a presentation by Dr. Trinity Bivalacqua discussing CORE-008, a phase 2, multi-arm, multi-cohort, open-label study to evaluate the safety and efficacy of cretostimogene grenadenorepvec in participants with high-risk non-muscle invasive bladder cancer (NMIBC). Treatment for patients with high-risk NMIBC consists of TURBT followed by intravesical BCG. Despite high initial response rates, over 50% of patients will recur and 20-40% are at risk for progression. Treatment of high-risk NMIBC is challenged by the BCG shortage and limited bladder-sparing options for patients who do not meet the strict definition of BCG-unresponsive disease by the US FDA. Thus, there exists a need for clinically effective, well-tolerated, and readily available treatment options for patients with high-risk NMIBC.
Cretostimogene grenadenorepvec is an oncolytic immunotherapy designed to selectively replicate in bladder cancer cells with Rb-E2F pathway alterations, commonly found in BCG-unresponsive high risk NMIBC. In addition, cretostimogene also expresses GM-CSF adding to local and systemic cancer control. The dual mechanism of action of cretostimogene is as follows:
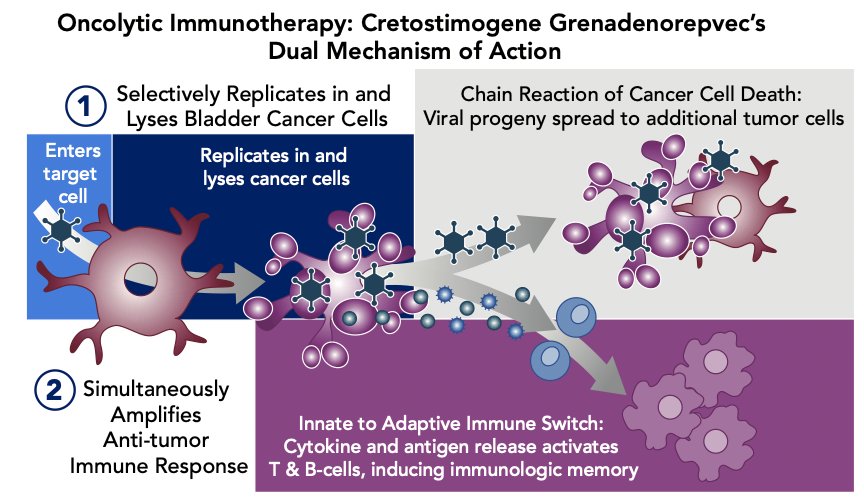
Cretostimogene has received Fast Track and Breakthrough Therapy Designations by the US FDA for the BCG-unresponsive high risk NMIBC with CIS indication secondary to the BOND-003 trial. Given the strength of recently presented data, the CORE-008 clinical trial was developed as a phase 2, multi-arm, multi-cohort trial to further evaluate the safety and efficacy of cretostimogene in both BCG-naive and BCG-exposed patients with high-risk NMIBC.
Eligibility criteria for CORE-008 require:
- Pathologic confirmation of high-risk NMIBC as defined by the AUA Guidelines
Age ≥18 years - ECOG performance status of 0-2
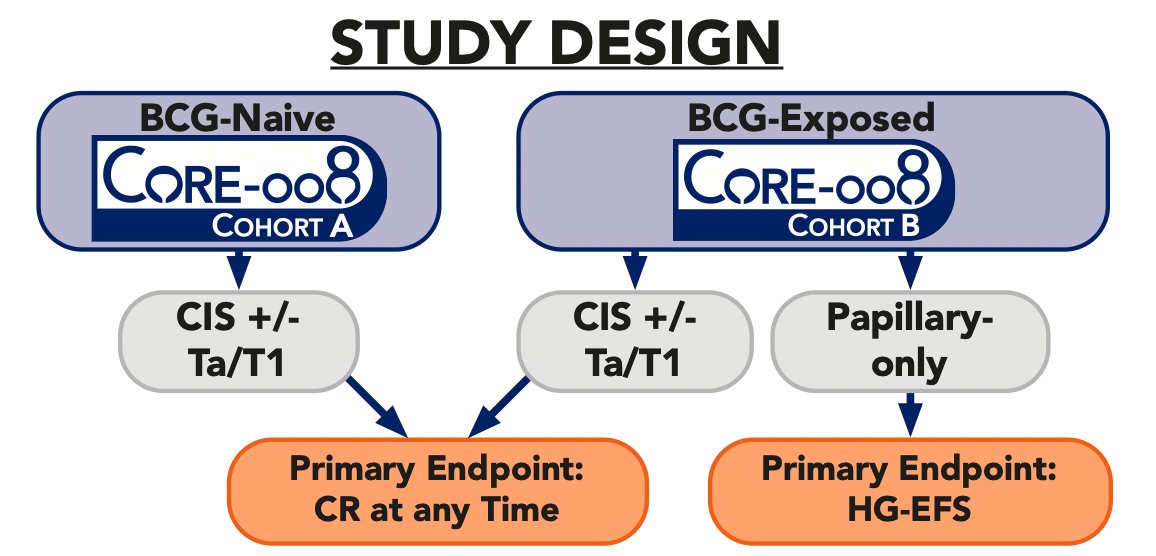
Cohort A (BCG-naive) will comprise CIS +/- HG Ta/T1 participants who have not received prior BCG. Cohort B (BCG-exposed) will consist of CIS +/- HG Ta/T1 or papillary-only patients who have received prior BCG and recurred either immediately after induction therapy (BCG-resistant) or recurred at a delayed time point, after adequate or inadequate BCG:
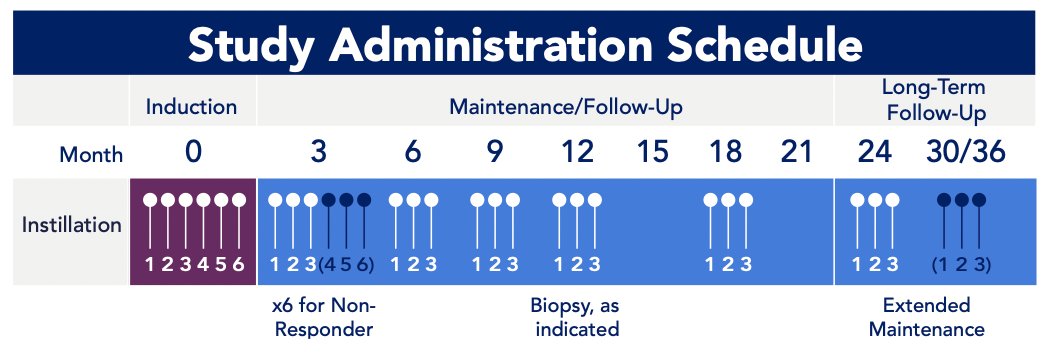
Intravesical cretostimogene will be instilled in combination with DDM, a transduction agent, adjuvant to TURBT for six weekly doses during the induction phase, followed by three weekly maintenance cycles quarterly through month 12, then every six months through month 36. Re-induction is permitted:
Primary disease assessments include serial cystoscopy with directed TURBT/biopsy (if indicated), urine cytology, axial imaging, and retrospective central review of pathologic samples. The primary endpoint for the CIS population is complete response at any time, and high-grade event free survival is the primary endpoint for papillary-only participants. Secondary endpoints will include:
- Duration of response
- All-cause event-free survival
- Bladder cancer specific survival
- Radical cystectomy free survival
- Safety
Exploratory outcome measures include health-related quality of life, overall survival, and biomarker assessments. Additional high-risk NMIBC cohorts are under development. Multiple clinical sites have been identified, the trial is in progress/actively enrolling, and Cohort B has received collaborative support from the SUO-CTC.
Presented by: Trinity J. Bivalacqua, MD, PhD, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, between the 3rd and 6th of December, 2024.
Related content: CORE-008 Cohort A Tests Oncolytic Immunotherapy in BCG-Naive High-Risk Bladder Cancer - Trinity Bivalacqua


