(UroToday.com) The 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX between December 3rd – 6th, 2024 was host to a bladder cancer poster session. Dr. Roger Li presented the results of an exploratory, ad hoc analysis of the phase II KEYNOTE-057 trial evaluating the post-treatment outcomes of patients with bacillus Calmette-Guérin (BCG)-unresponsive, high-risk (HR), non-muscle invasive bladder cancer (NMIBC) who experienced a ‘non-response’ to pembrolizumab monotherapy.
Radical cystectomy remains the recommended standard of care treatment for BCG-unresponsive HR NMIBC, although many patients decline or are unable to undergo surgery and elect bladder-sparing treatment instead.1,2 The phase II KEYNOTE-057 trial (NCT02625961) demonstrated that pembrolizumab monotherapy can serve as a bladder-sparing treatment option for patients with BCG-unresponsive HR NMIBC with carcinoma in situ (CIS) with or without papillary tumors (cohort A), or with papillary tumors without CIS (cohort B) who decline or are unable to undergo radical cystectomy.3,4 Outcomes, especially those related to disease progression, of patients who experienced non-response to bladder-sparing treatment, including pembrolizumab, are of interest as they may inform clinicians of treatment strategies and decisions as well as the design of future clinical trials
The study objective was to describe clinical outcomes and characteristics of participants with BCG-unresponsive NMIBC who experienced persistent, recurrent, or progressive disease (PD) despite pembrolizumab monotherapy and subsequently received either RC and/or other bladder-sparing treatments or no subsequent therapy in the pooled (cohorts A and B) KEYNOTE-057 population.
The study design of KEYNOTE-057 is illustrated below: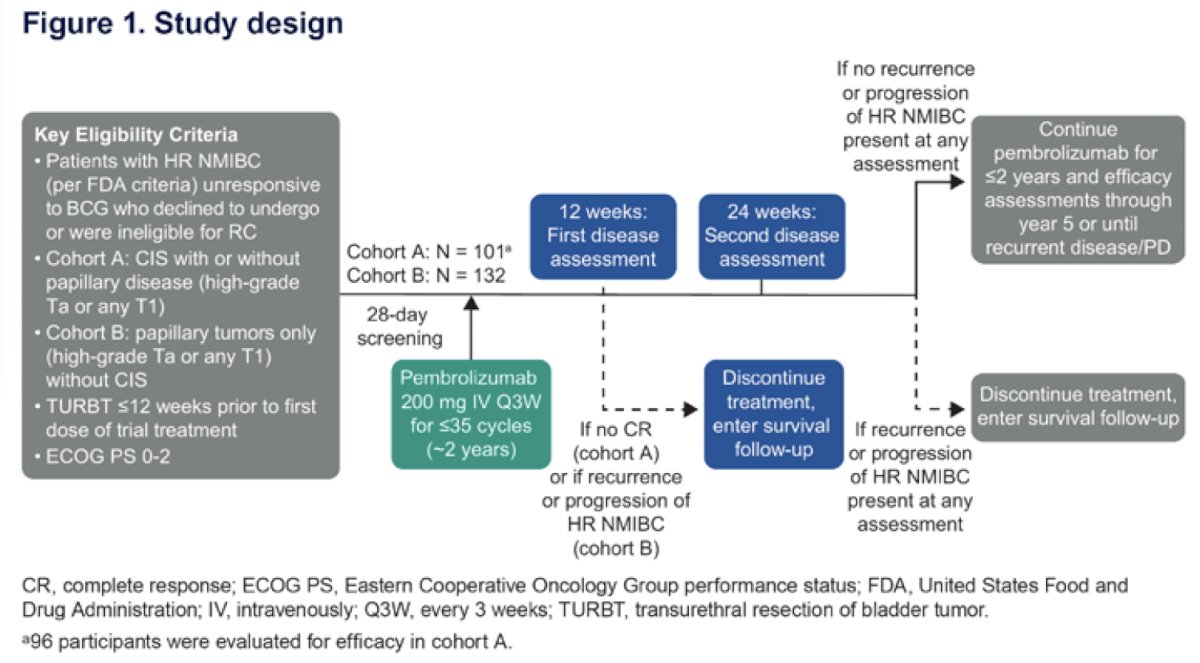
Study participants were categorized into one of three groups:
- Early radical cystectomy: Participants who received radical cystectomy within 4 months of confirmed treatment failure
- Delayed radical cystectomy: Participants who received surgery >4 months after treatment failure confirmation or received other bladder-sparing treatment before radical cystectomy
- No radical cystectomy: Participants who received no radical cystectomy (including participants who received bladder-sparing treatment alone or had no reported subsequent therapy after confirmed nonresponse to pembrolizumab)
The study endpoints were as follows:
- Time from the date of confirmed non-response to pembrolizumab (date of pathological confirmation or the start date of efficacy follow-up) to radical cystectomy or bladder-sparing treatment, whichever occurred first
- Confirmed nonresponse to pembrolizumab was defined as persistence or recurrent high-grade NMIBC or progression to muscle-invasive bladder cancer (MIBC) or metastatic disease despite pembrolizumab treatment
- Progression-free survival (PFS: time from the date of confirmed nonresponse to pembrolizumab to progression to MIBC or metastatic disease or death due to PD, whichever occurred first)
- Metastasis-free survival (MFS; time from the date of confirmed nonresponse to pembrolizumab to distant recurrence or death due to PD, whichever occurred first)
- Overall survival (OS; time from the date of confirmed nonresponse to pembrolizumab to death from any cause)
- PFS in participants with recurrence within 4 months or 24 months of pembrolizumab initiation
- Pathological outcomes (per investigator report)
PFS, MFS, and OS were estimated using the Kaplan-Meier method. No formal hypothesis testing was performed. The data cutoff dates were May 30, 2023 (cohort A) and October 20, 2022 (cohort B).
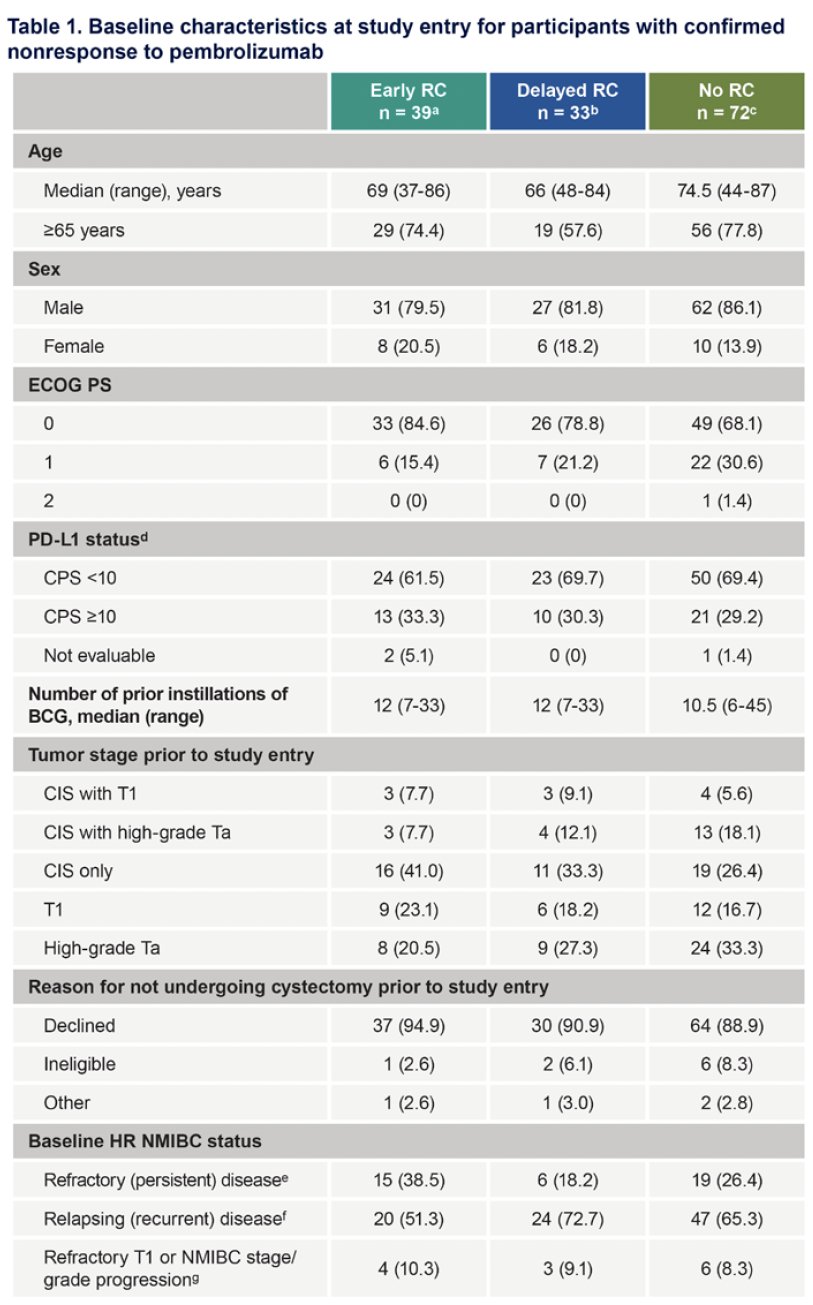
The study cohort included a total of 144 participants with a non-response to pembrolizumab:
- Early radical cystectomy: 39 patients
- Delayed radical cystectomy group: 33
- No radical cystectomy: 72
The baseline patient characteristics are summarized below. The patients who did not undergo a cystectomy were older (75 years versus 66–69 years) and had a worse ECOG performance status (1-2: 32% versus 15-21%). Patients in the early cystectomy group were more likely to have refractory (i.e. persistent) disease: 39% versus 18–26%.
The time from confirmed pembrolizumab non-response to radical cystectomy or subsequent therapy is summarized below. The time from pembrolizumab non-response to subsequent therapy was numerically longer in participants who did not undergo a radical cystectomy or received other subsequent therapy, compared with those who underwent early or delayed surgery.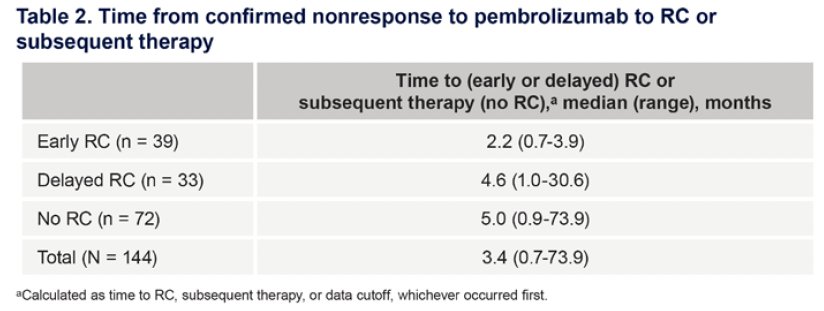
The 36-months PFS rate was 86% in the no radical cystectomy group, compared to 72% and 68% in the early and delayed surgical groups. Metastasis-free survival rates were also superior in the no radical cystectomy group (at 36 months: 93% versus 87% and 79%, respectively). The highest overall survival rates were in the delayed surgical group, which may reflect a survival bias (i.e., patients had to survive for a certain timeframe before becoming eligible for inclusion in the study subgroup).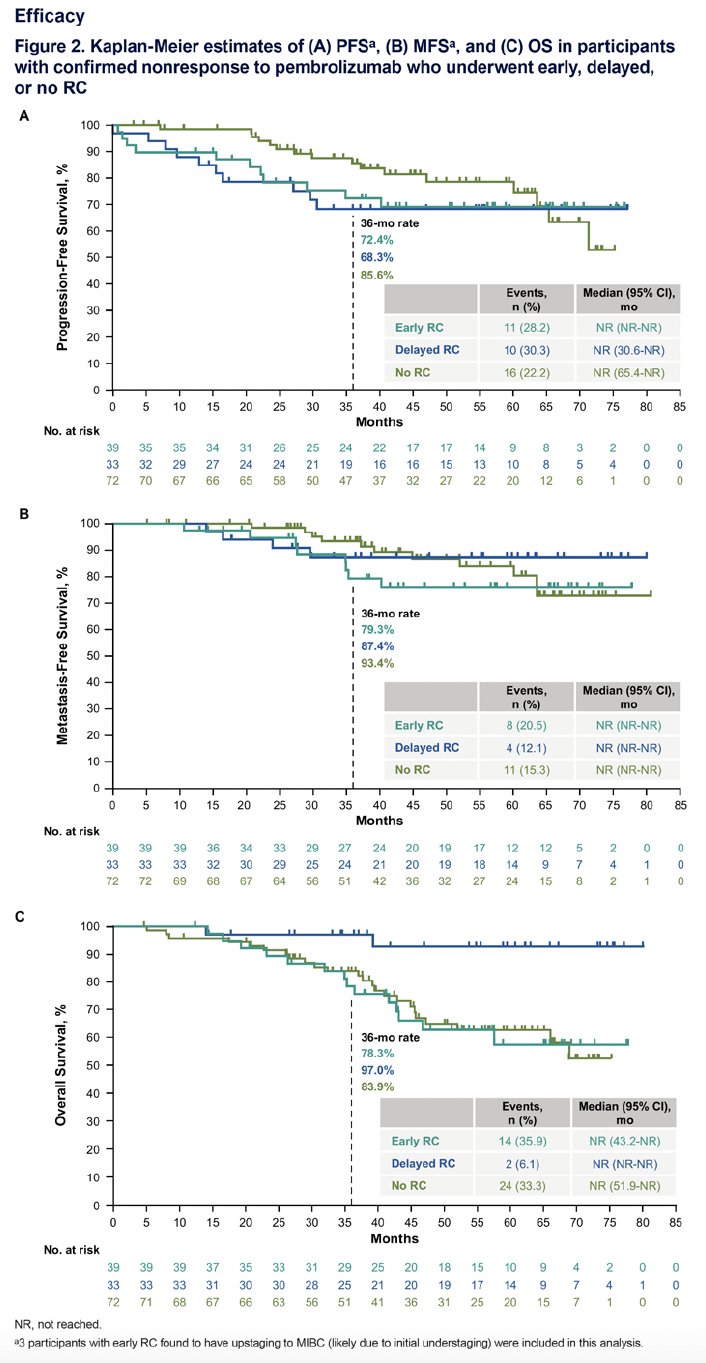
There were no marked differences in PFS rates among participants who experienced disease recurrence within 4 months versus ≥4 months of pembrolizumab initiation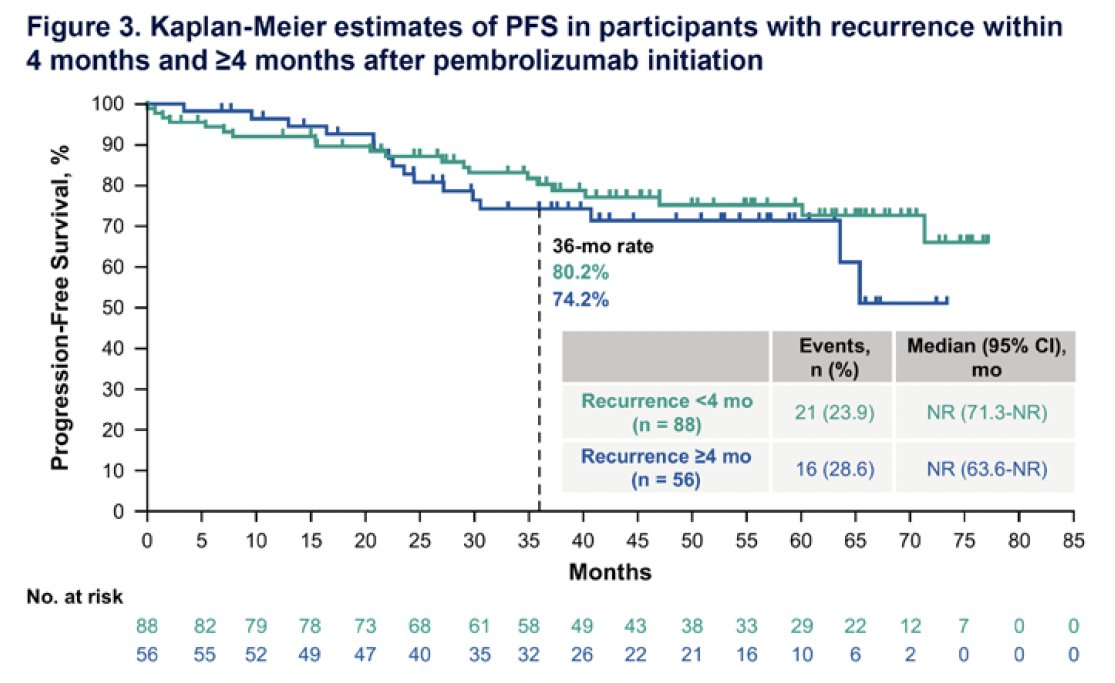
PFS was not markedly different based on duration of bladder-sparing treatment in participants who underwent delayed versus no radical cystectomy.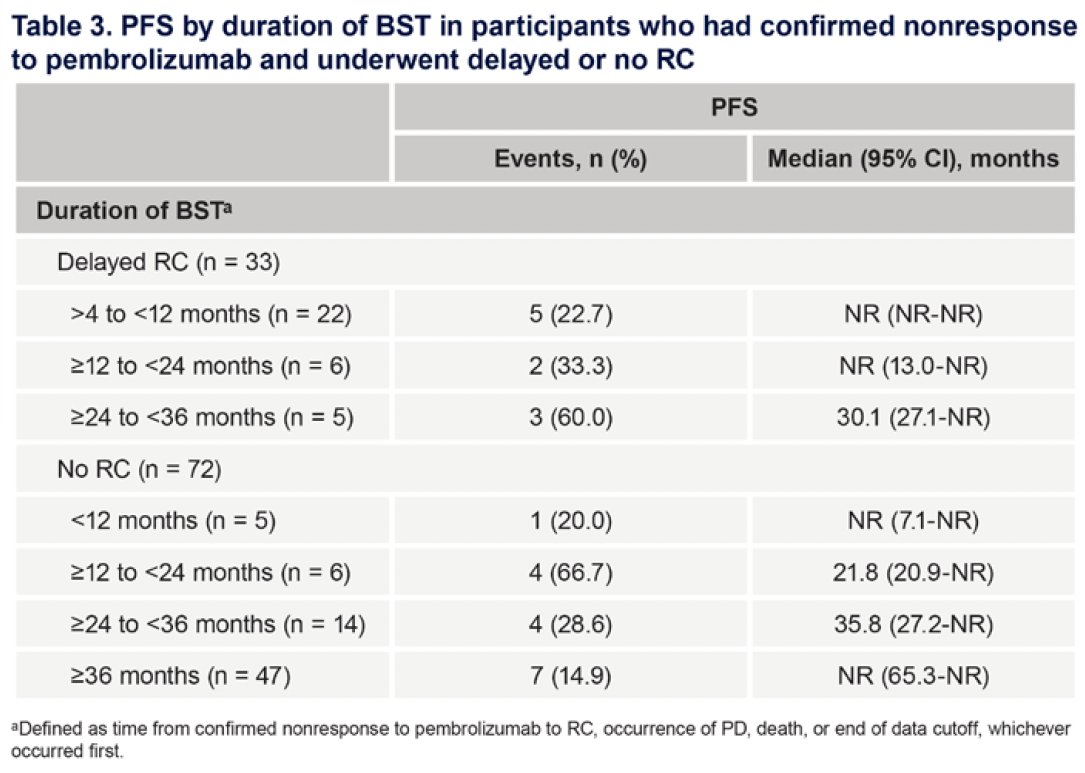
Participants who underwent early versus delayed radical cystectomy had similar pathological outcomes.
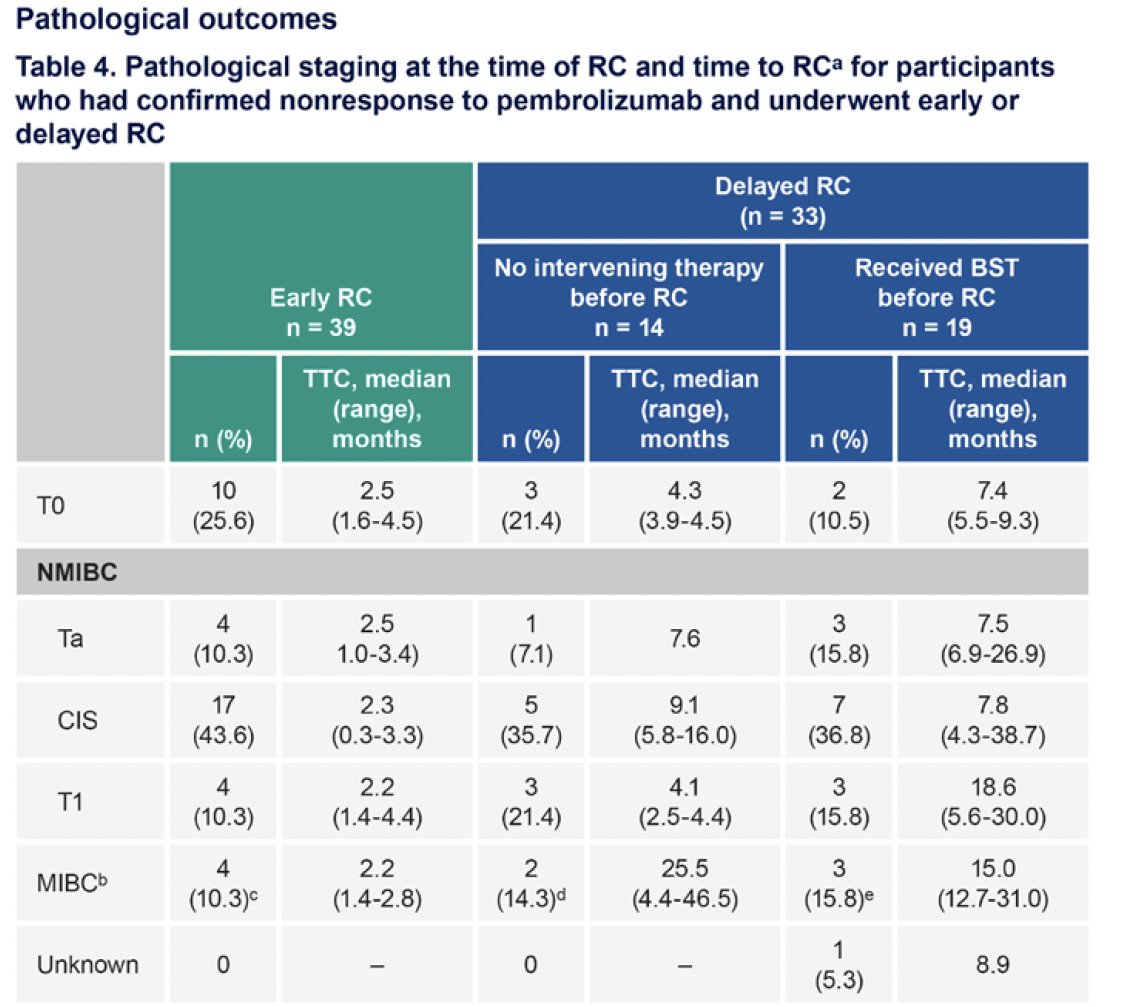
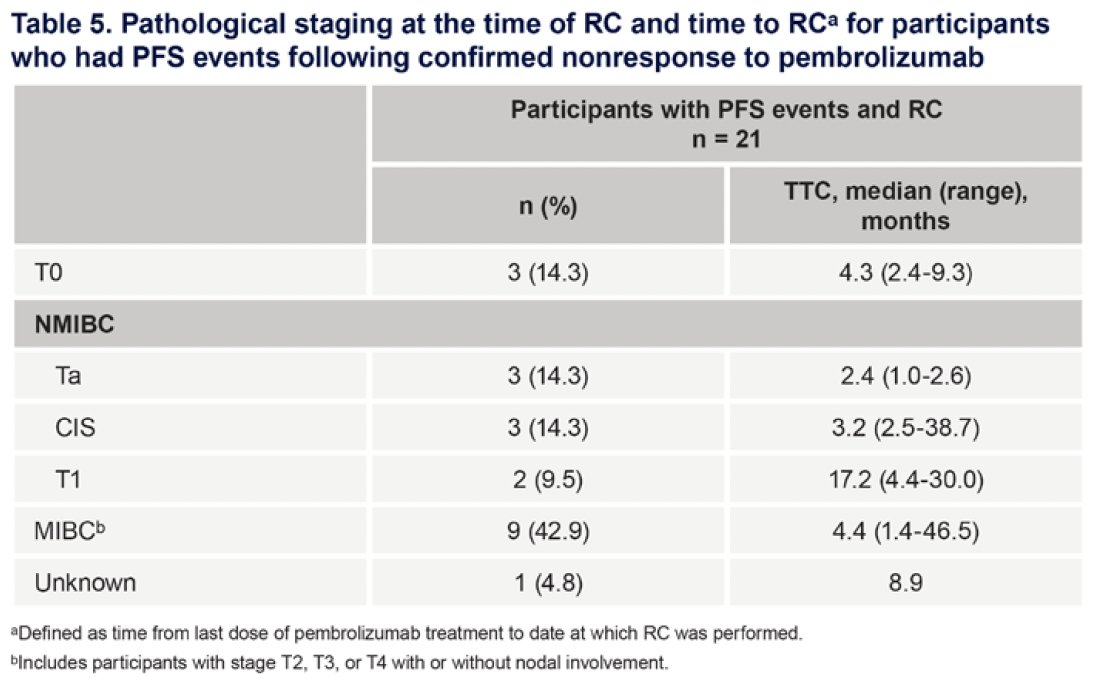
Dr. Li concluded his presentation as follows:
- Clinical outcomes, including PFS, MFS, and OS, were generally similar between participants with HR NMIBC who underwent early radical, delayed, or no radical cystectomy after confirmed pembrolizumab non-response
- The time from pembrolizumab non-response to subsequent therapy was numerically longer in participants who did not undergo a radical cystectomy or received other subsequent therapy, compared with those who underwent early or delayed surgery
- PFS was not markedly different based on duration of bladder-sparing treatment in participants who underwent a delayed or no radical cystectomy, or between participants who experienced disease recurrence within 4 months versus ≥4 months of pembrolizumab initiation in all participants
- Participants who underwent early versus delayed radical cystectomy had similar pathological outcomes
- The results should be interpreted with caution due to the post hoc nature of this analysis, small sample size of the subgroups, and because disease characteristics, prognosis, or participant and physician opinion may have influenced whether participants underwent radical cystectomy or bladder-sparing treatment
- Overall, data from this post hoc analysis suggest that implementing other second-line bladder-sparing therapy after pembrolizumab nonresponse may be a feasible second-line option to delay or avoid radical cystectomy
Presented by: Roger Li, MD, Urologic Oncologist, Moffitt Cancer Center, Tampa, FL
Written by: Rashid K. Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX between December 3rd and 6th, 2024
References:- Flaig TW, Spiess PE, Agarwal N, et al. NCCN Guidelines Insights: Bladder Cancer. J Natl Compr Canc Netw. 2022;20:866-878.
- Gontero P, De Nunzio C, Bientinesi R, et al. Radical cystectomy and perioperative management in non-metastatic muscle-invasive bladder cancer patients: EAU guidelines. Eur Urol. 2023; S0302-2838(24):02514-4.
- Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2021; 22:919-930.
- Necchi A, Raggi D, Gallina A, et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive bladder cancer (PURE-01): an open-label, single-arm, phase 2 study. Lancet Oncol. 2024;25: 720-730.


