(UroToday.com) The 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX between December 3rd – 6th, 2024 was host to a bladder cancer poster session. Dr. Vikram Narayan presented the results of ABLE-32, a randomized, controlled, phase IIIB clinical trial of Nadofarogene Firadenovec-vcng versus observation in patients with intermediate-risk, non-muscle invasive bladder cancer (NMIBC).
The AUA/SUO guidelines currently define intermediate-risk NMIBC as recurrent LG Ta within 1 year, solitary LG Ta >3 cm, multifocal LG Ta, HG Ta (≤3 cm), and/or LG T1 disease.1 Nadofaragene firadenovec-vncg is the first US FDA–approved intravesical gene therapy for the treatment of high-risk BCG–unresponsive NMIBC with CIS ± papillary tumors.2 Nadofaragene firadenovec is a non-replicating and non-integrating adenoviral vector–based gene therapy that delivers the human IFNα2b gene to urothelial cells, and Syn3, to enhance viral transduction of the urothelium.3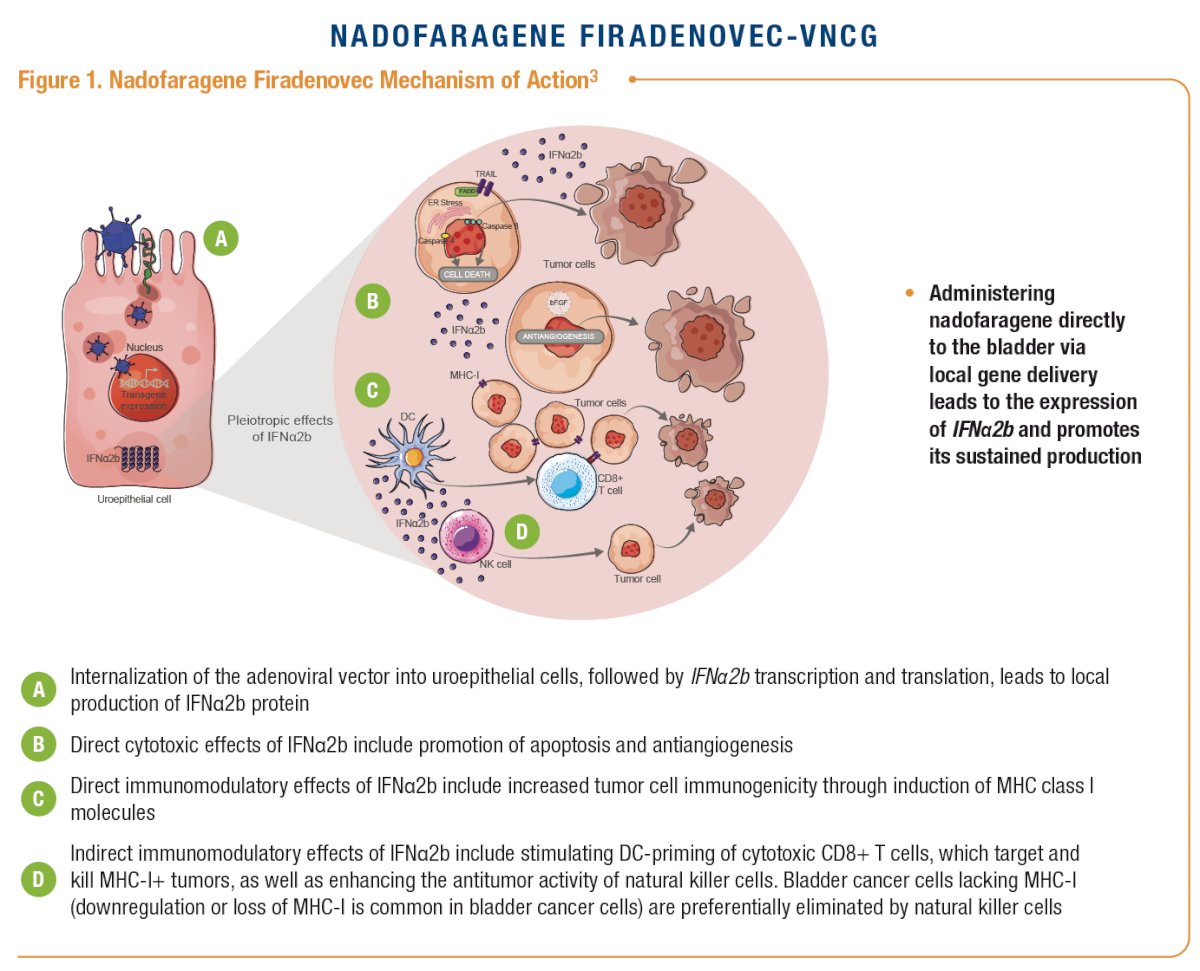
In a nonrandomized, multicenter, open-label, repeat-dose, phase III study, 53.4% of participants (55/103) with CIS ± HG Ta/T1 achieved a complete response 3 months after the first instillation. Nadofaragene firadenovec was well tolerated, with no grade 4 or 5 study drug–related adverse events. ABLE-32 (NCT06510374) is an ongoing, open-label, randomized, controlled, phase IIIb study to evaluate the efficacy of nadofaragene firadenovec administered every 3 months versus observation in participants with intermediate-risk NMIBC.
The study design is illustrated below:
In this trial, 454 intermediate-risk NMIBC patients (newly diagnosed or recurrent) will undergo 1:1 randomization, following a TURBT +/- peri-operative chemotherapy, to nadofaragene firadenovec 75 ml (3x1011 vp/mL) instilled quarterly versus observation. Notably, patients may crossover at recurrence of intermediate-risk NMBIC, if this occurs within 24 months.
The primary study endpoint is recurrence-free survival, defined as the time from the date of randomization to first documented recurrence (low-, intermediate-, or high-risk) or progression (high-risk or muscle-invasive) or death (due to any cause), whichever occurs first.
Key secondary endpoints include recurrence-free survival at 12 and 24 months and safety (frequency and severity of adverse events). Exploratory endpoints include recurrence-free survival up to year 5 from randomization and changes in the expression of potential biomarkers in urine and blood. Participants are expected to be observed through 60 months.
To analyze recurrence-free survival, a stratified log-rank test will be conducted to test the null hypothesis of identical hazard functions (of the nadofaragene firadenovec arm relative to the observation arm) within every stratum compared with the alternative hypothesis of
a lower hazard function for the nadofaragene firadenovec arm in at least 1 stratum. Safety analyses will be descriptive.
The ABLE-32 study milestones are summarized below:
Presented by: Vikram Narayan, MD, Assistant Professor, Department of Urology, Emory University, Winship Cancer Institute, Atlanta, GA
Written by: Rashid K. Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX between December 3rd and 6th, 2024
References:- Chang SS, Boorjian SA, Chou R, et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J Urol. 2016;196(4):1021-1029. doi:10.1016/j.juro.2016.06.049.
- ADSTILADRIN® (nadofaragene firadenovec-vncg). Prescribing information. Ferring Pharmaceuticals Inc.; August 2024.
- Narayan VM, Shi J, Torrens H, et al. Advances in Targeted Therapies for Solid Tumors. Front Oncol. 2024;14:1359725. doi:10.3389/fonc.2024.1359725.


