(UroToday.com) The 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, between December 3 and December 6, 2024, was host to the Bladder Cancer Session II. Dr. Mamta Parikh discussed Emerging Therapeutics: Combination Therapy in the Neoadjuvant Setting for Bladder Cancer.
Dr. Parikh began her presentation by emphasizing that the neoadjuvant setting in muscle-invasive bladder cancer (MIBC) is rapidly evolving, making significant progress this year. She noted that patients eligible for or choosing radical cystectomy (RC) could be divided into two groups: cisplatin-eligible and cisplatin-ineligible. Historically, treatment options for cisplatin-eligible patients included gemcitabine-cisplatin or ddMVAC, while there was no established regimen for cisplatin-ineligible patients. Below is a comprehensive summary of the current and ongoing studies providing treatment options for both groups.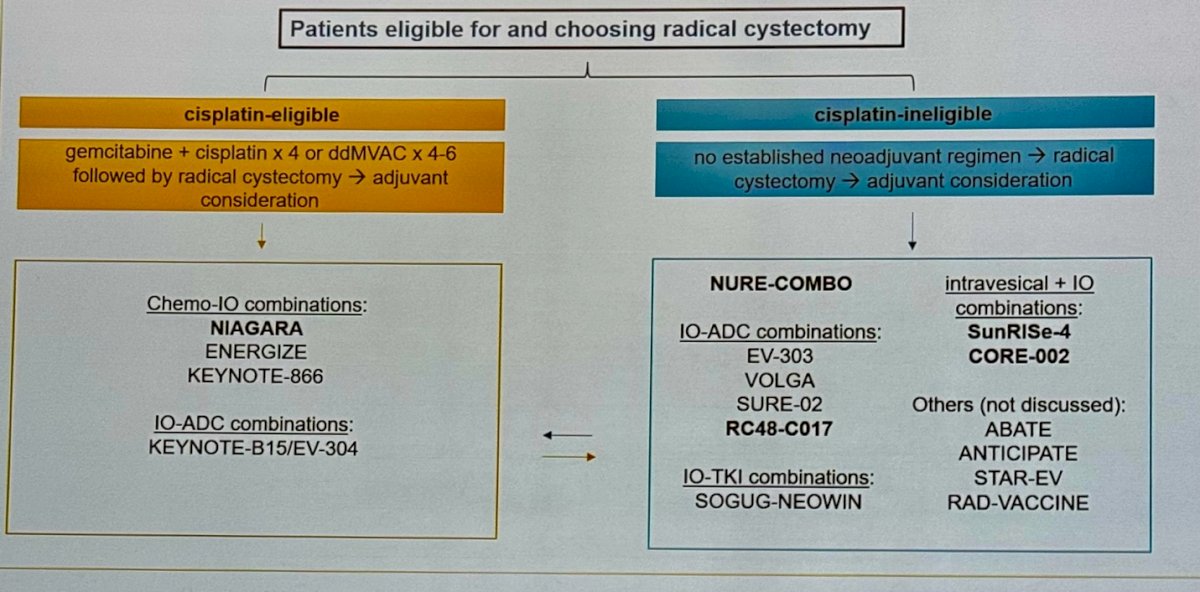
Cisplatin-eligible perioperative trials
The NIAGARA study was the first phase 3 trial testing perioperative immune checkpoint inhibitor (Durvalumab) combined with NAC in cisplatin-eligible patients with muscle-invasive bladder cancer (MIBC).
The NIAGARA trial enrolled adult patients with cisplatin-eligible MIBC (cT2-TaN0/1M0), including those with divergent differentiation or histologic subtypes. Notably, the cisplatin eligibility was adjusted to a CrCl of ≥40 mL/min. In the Durvalumab arm, patients were randomized to receive Durvalumab with Gemcitabine + Cisplatin (GC) for 4 cycles, followed by RC and adjuvant Durvalumab for up to 8 cycles. In the comparator arm, patients were randomized to receive GC followed by RC and did not receive any adjuvant treatment. The primary endpoints were Event-free survival (EFS), and pathological complete response rate (pCR), evaluated by blinded central pathology review (BCPR). The study design is shown below: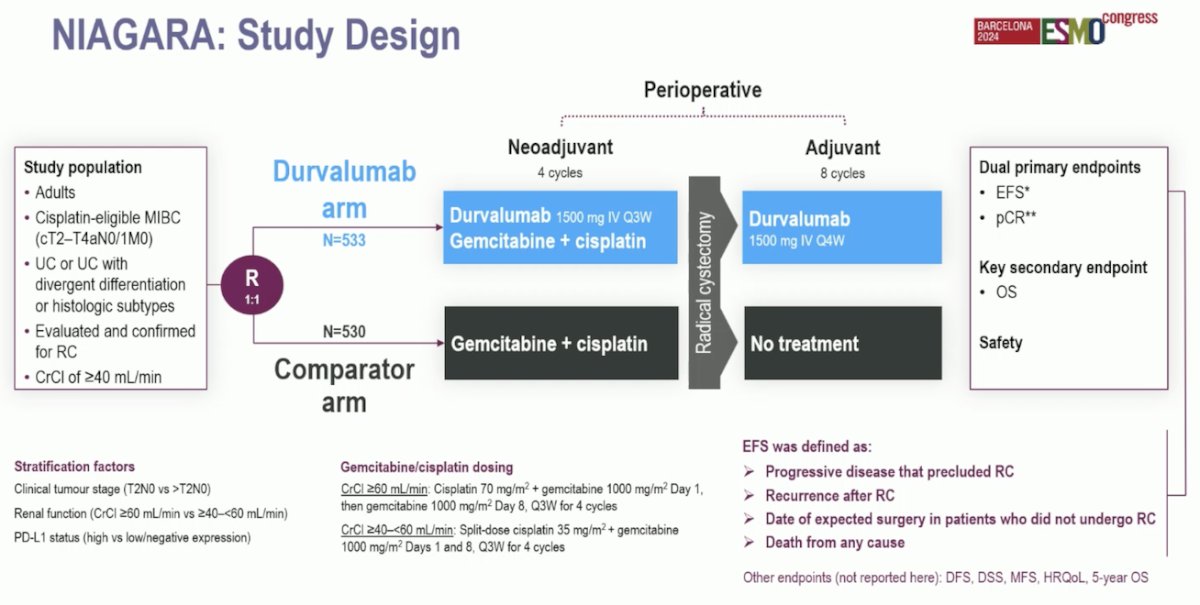
The NIAGARA study found significantly better outcomes in the dual co-primary endpoints for patients treated with Durvalumab in addition to gemcitabine and cisplatin. The pathological complete response (pCR) was notably higher at 33.8% in the Durvalumab arm compared to 25.8% in the comparator arm. Additionally, the event-free survival (EFS) at 2 years was significantly improved, with 67.8% in the Durvalumab arm versus 59.8% in the comparator arm. The hazard ratio (HR) for EFS was 0.68.1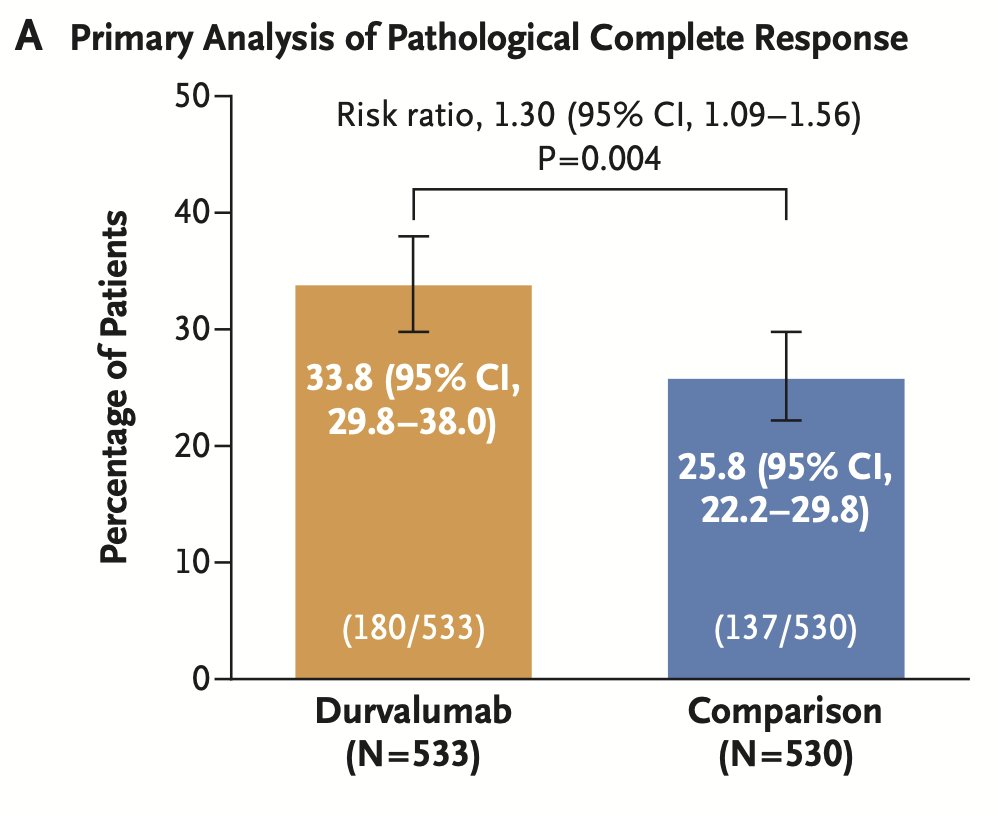
There are three other studies examining the combination of chemotherapy and immune checkpoint inhibitors. The first is EV-304, a randomized phase 3 study of perioperative enfortumab vedotin plus pembrolizumab (4 cycles preoperatively and 13 cycles postoperatively) versus chemotherapy in cisplatin-eligible patients with MIBC. The study design is shown below.
The Keynote-866 is a phase 3 study involving cisplatin-eligible patients. This study compares two regimens: the first regimen includes 4 cycles of neoadjuvant pembrolizumab + GC followed by 13 cycles of adjuvant pembrolizumab after RC. The second regimen consists of 4 cycles of neoadjuvant placebo + GC followed by 13 cycles of adjuvant placebo after RC. 
Lastly, the third study is the ENERGIZE study. This study has three arms, randomizing cisplatin-eligible patients to GC (Arm A), GC + Nivolumab followed by RC and adjuvant Nivolumab + linrodostat placebo (Arm B), and GC + Nivolumab followed by RC and adjuvant Nivolumab + linrodostat (Arm C). 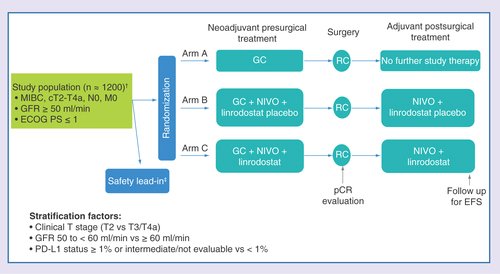
All these studies have the sandwich approach, but Dr Parikh highlighted that in all of them including NIAGARA, the control arm after RC goes to observation or receives placebo and does not receive previous standard of care adjuvant treatment (Nivolumab, Pembrolizumab).
Cisplatin-ineligible perioperative trialsThe NURE-COMBO study used a perioperative approach for cisplatin ineligible patients, This trial included patients who were Cisplatin unfit or declined cisplatin-based chemotherapy and had previously untreated MIBC (cT2-4aN0-1M0, as per CT and MRI scan) and with predominant (>50%) urothelial carcinoma histology, Patients were scheduled to receive four cycles of Nab Paclitaxel followed by radical cystectomy and 13 cycles of adjuvant nivolumab. 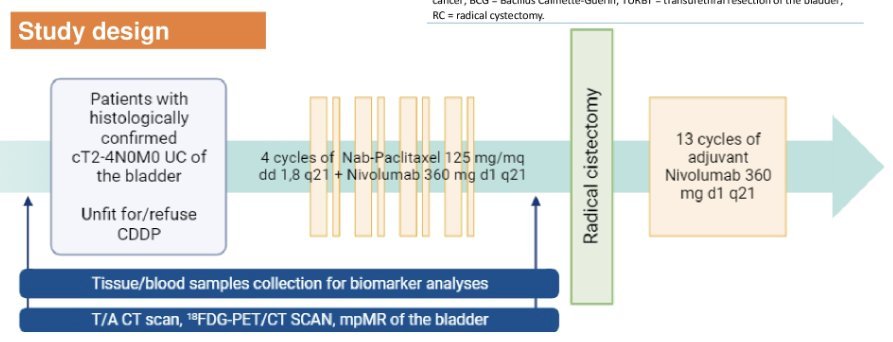
The investigators enrolled 31 patients, of which 16% could not complete NAC due to adverse events, and found a pCR of 32.3 with a 12month event free-survival of 90%.
Another study in this space is the EV-303, which employs a sandwich approach and compares three perioperative strategies: neoadjuvant pembrolizumab alone, observation, and pembrolizumab + EV. All are followed by RC and then either adjuvant pembrolizumab, observation, or pembrolizumab + EV in the adjuvant setting, as shown below: 
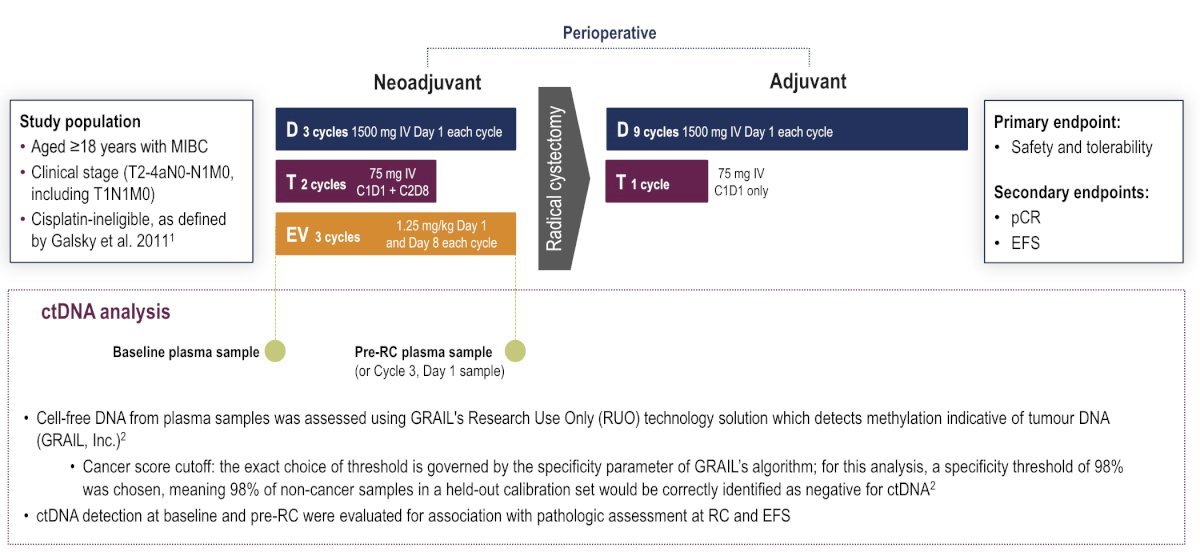
The VOLGA study also in patients with cisplatin-ineligible MIBC is studying a sandwich approach of perioperative treatment. In this study Patients received 3 cycles of neoadjuvant therapy every 3 weeks: durvalumab (1500 mg; day 1 each cycle) + tremelimumab (75 mg; day 1 cycle 1, and day 8 cycle 2) + enfortumab vedotin (1.25 mg/kg; days 1 and 8 each cycle), followed by radical cystectomy, then 9 cycles of adjuvant durvalumab (every 4 weeks; day 1 each cycle) and tremelimumab (day 1, cycle 1 only). The trial design for VOLGA is as follows:
The SURE-02 and SURE-01 trials are perioperative studies investigating an antibody-drug conjugate (ADC) targeting TROP-2, Sacituzumab govitecan. Eligible patients will receive 4 cycles of 10 mg/kg sacituzumab govitecan IV on days 1 and 8 of each 21-day cycle (SURE-01) or sacituzumab govitecan plus pembrolizumab on day 1 every 21 days at the standard dose of 200 mg intravenously (SURE-02). In SURE-01, a pathologic complete response was observed in 37.5% of patients who underwent a radical cystectomy. For SURE-02 we are awaiting results and would help to determine if the payload in this ADCs makes a difference. 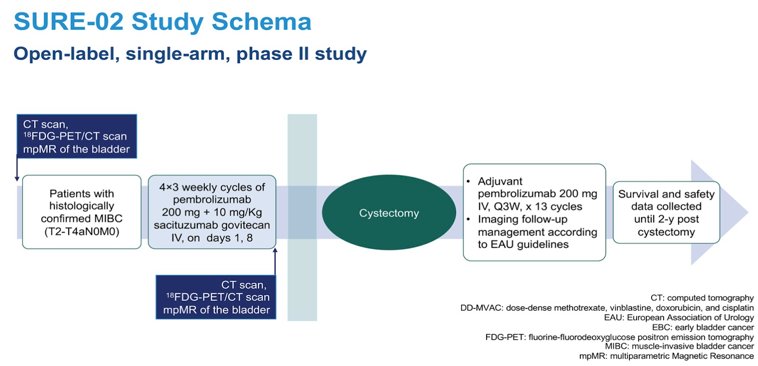
Other targeted approaches with ADCs include the RC48-C017 study assessing neoadjuvant disitamab vedotin and anti-HER2 ADC. Eligible patients received DV + Toripalimab for 6 cycles followed by RC and adjuvant Toripalimab for up to 20 cycles. The pCR was 61.3% in the patients who underwent RC, in the patients who were HER2 IHC 3+ the pCR was 83%.

Erdafitinib targets FGFR2/3 mutations and has been introduced into the neoadjuvant space in the SOGUG-NEOWIN study. Eligible patients are those with cT2-4aN0-1M0 urothelial carcinoma who test positive for FGFR mutations and are ineligible for or refuse neoadjuvant cisplatin. Ninety patients will be randomized to daily erdafitinib monotherapy versus combination erdafitinib + cetrelimab. The co-primary study endpoints are pathologic complete response and downstaging.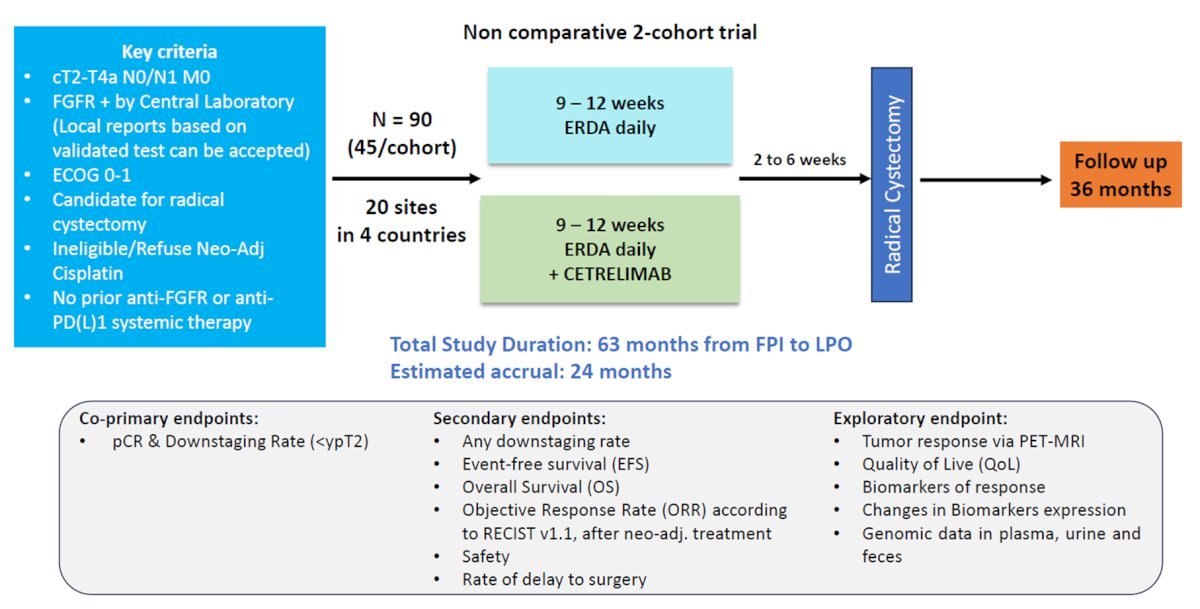
The SunRIse-4 Study included patients with cT2-4aN0M0 MIBC, Predominant urothelial carcinoma histology, Ineligible for or refusing NAC, ECOG performance status 0–1. Patients underwent 5:3 randomization to: TAR-200 225 mg gemcitabine every 3 weeks (indwelling) for 12 weeks + cetrelimab intravenously for 12 weeks (4 cycles) or Cetrelimab intravenously for 12 weeks (4 cycles) followed by RC.
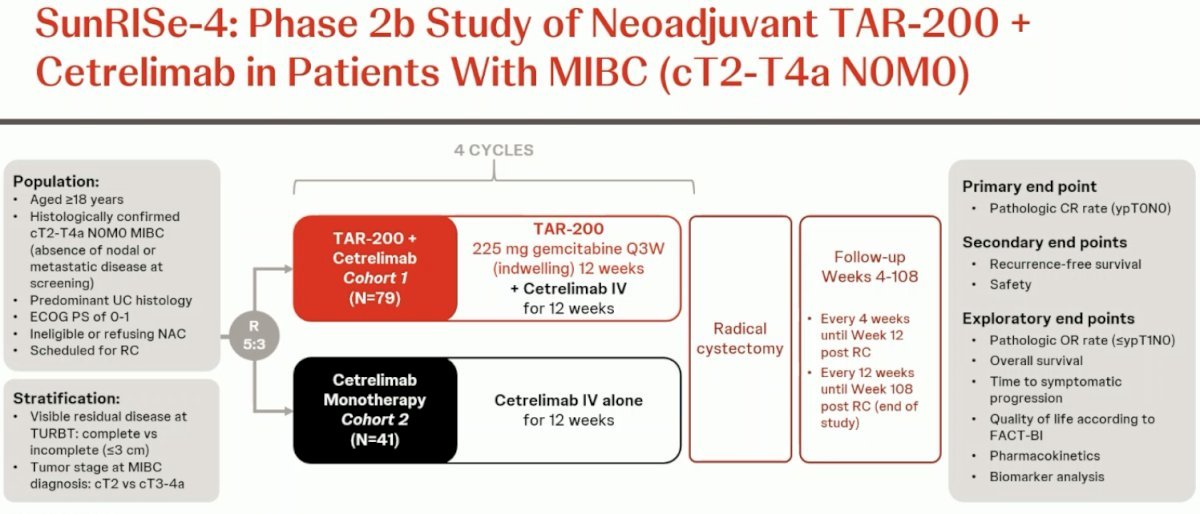
In efficacy evaluable patients, the pCR was 42% (95% CI, 28-56) for the combination (n=53) versus 23% (95% CI, 10-41) for cetrilimab alone (n=31). Clinical staging and the quality of TURBT may influence efficacy, although the sample size remains small. Regarding safety Treatment-related adverse events (TRAEs) occurred in 72% of patients, with serious adverse events (SAEs) reported in 11% and discontinuation due to TRAEs occurred in 13% of patients.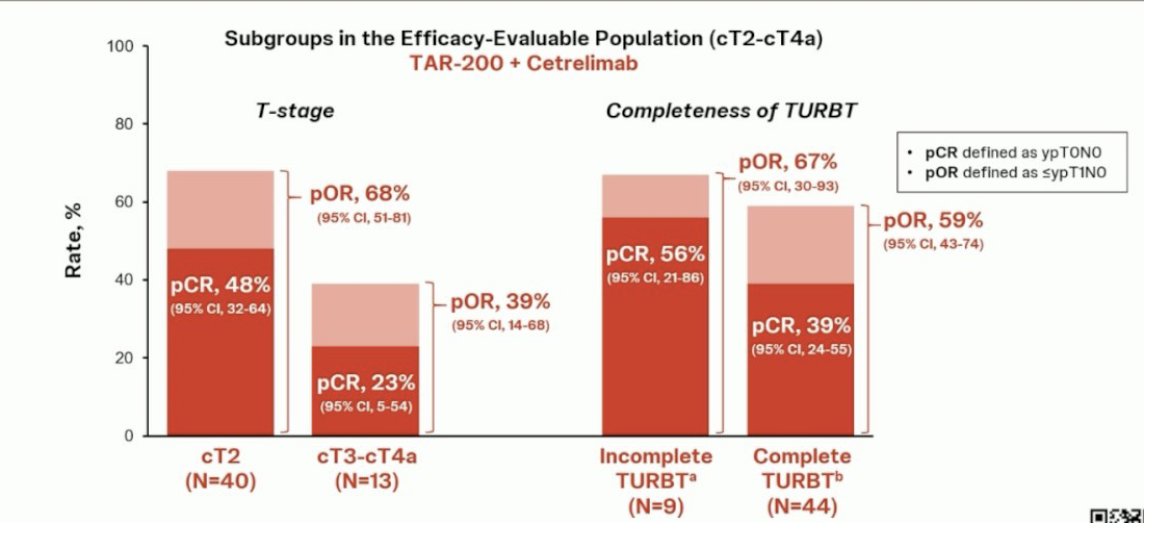
Moreover, the CORE-002 study assessing GC0070 (Cremostigene) + Nivolumab in the neoadjuvant setting, enrolled Patients with MIBC cisplatin ineligible who underwent TURBT, received intravesical cremostigene weekly x 6 and IV Nivolumab every 4 weeks.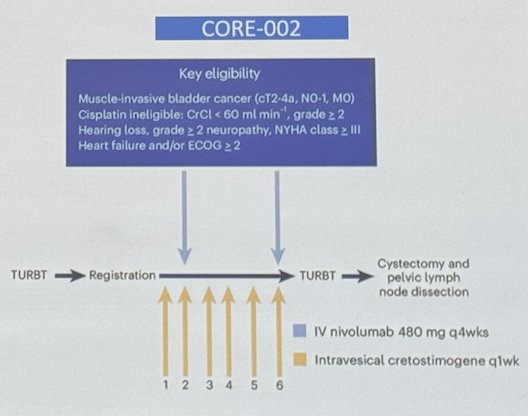
The study enrolled 21 patients and reported a pCR of 42% and 1-year RFS of 70% with tolerable toxicity.
Dr. Parikh concluded her presentation summarizing the current state of neoadjuvant combination therapy is:
For cisplatin-eligible patients
- NIAGARA: positive trial, but perioperative approach remains an open question
- Other Phase 3 studies in this space (KN-866, ENERGIZE, EV-304) designed similarly would leave us with the same questions.
For cisplatin-ineligible patients
- Closer to reality- Phase 3 trials are landmarks (EV-303, VOLGA)
- Intravesical - Immunotherapy combinations trials
- There could be a role for personalizing treatment using ADCs (Nectin-4, TROP-2, HER2) FGFR2/3.
Presented by: Mamta Parikh, MD, MS, Associate Professor, Division of Hematology and Oncology at UC Davis Comprehensive Cancer Center, Sacramento, CA
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, between the 3rd and 6th of December, 2024.
References:Related Content: Emerging Neoadjuvant Combinations for Muscle-Invasive Bladder Cancer - Mamta Parikh


