(UroToday.com) The 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, between December 3 and December 6, 2024, was host to the Abstract/Posters Session. Dr. Eric Jonasch presented a post hoc pooled analysis of four clinical trials evaluating the safety of belzutifan in patients with renal cell carcinoma (RCC).
Dr. Jonasch began his poster presentation by highlighting that Belzutifan, the first-in-class hypoxia-inducible factor-2α (HIF-2α) inhibitor. HIF-2α is a transcription factor that plays a critical role in the cellular response to low oxygen levels (hypoxia). Under normal conditions, HIF-2α is degraded, but in certain diseases like von Hippel-Lindau (VHL) disease and some cancers such as renal cell carcinoma (RCC), HIF-2α is stabilized and accumulates, leading to the activation of genes that promote angiogenesis, cell proliferation, and survival. The figure below highlights Belzutifan mechanism of action: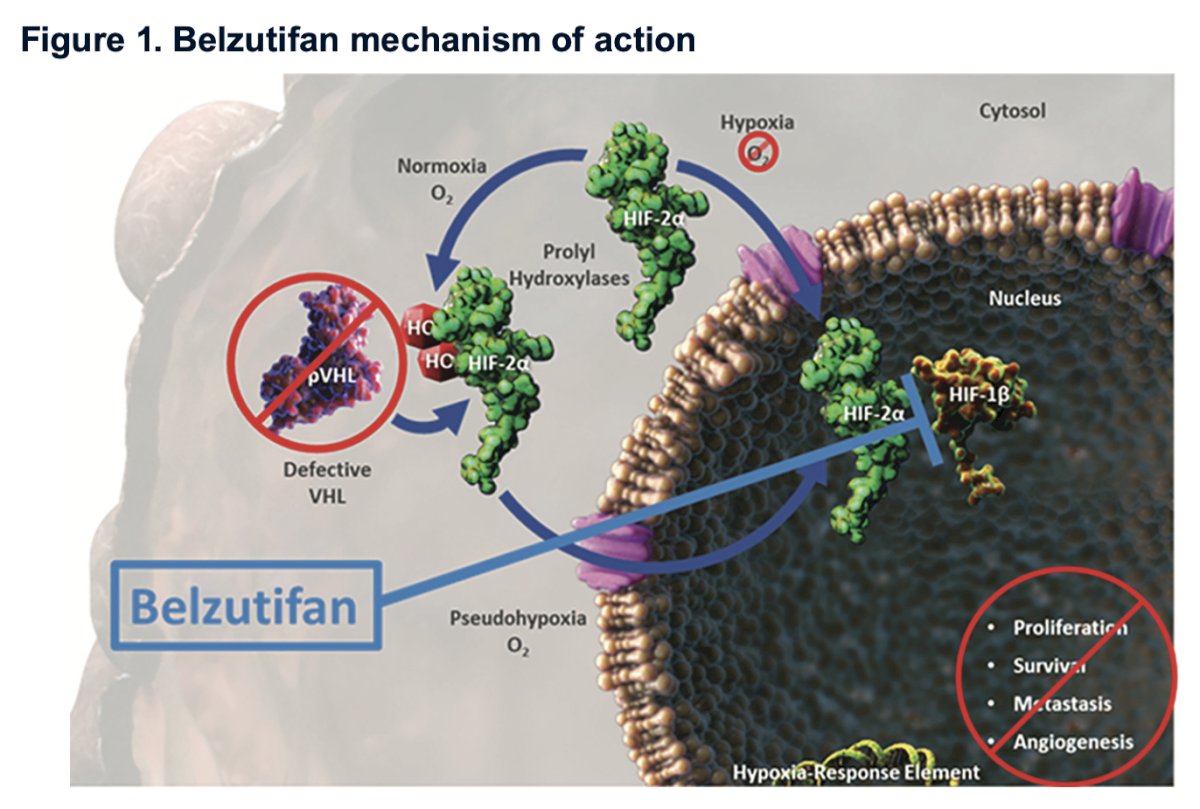
Belzutifan is approved in multiple countries, including the United States and Canada, for the treatment of patients with VHL disease–associated neoplasms. In the United States, it is also approved for adult patients with advanced renal cell carcinoma (RCC) who have previously been treated with a PD-(L)1 inhibitor and a vascular endothelial growth factor-tyrosine kinase inhibitor (VEGF-TKI). Several studies have evaluated Belzutifan in patients with advanced RCC. Dr. Jonasch presented the results of a post hoc pooled analysis of Belzutifan safety in patients with RCC who received Belzutifan 120 mg QD across the LITESPARK-001, LITESPARK-004, LITESPARK-005, and LITESPARK-013 studies.1-3
The study design is outlined in the figure below. Briefly, the investigators included patients who received ≥1 dose of belzutifan 120 mg by mouth QD across the 4 studies and had either a diagnosis of advanced clear cell RCC with or without VHL disease. The pooled population comprised 576 patients, with 66% of them coming from the LITESPARK-005 trial. 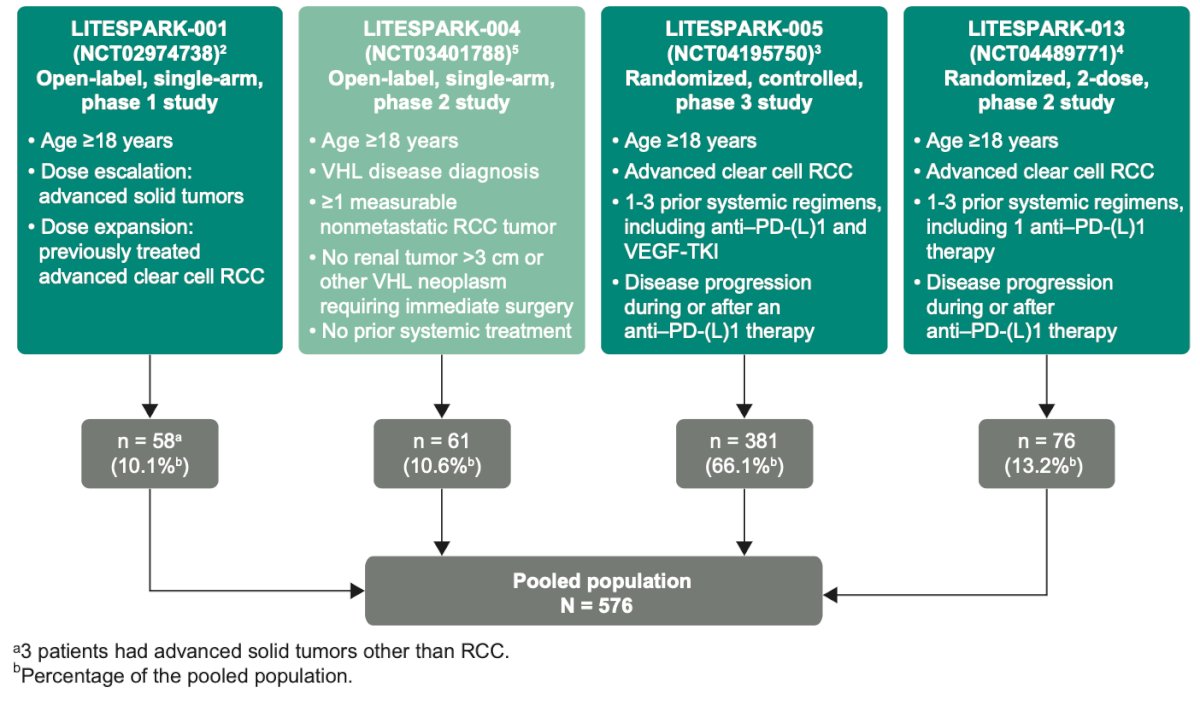
Table 1 shows the baseline characteristics of the pooled population (n=576). Notably, the median age was 61 years, with most patients being male (77%) and having an ECOG-PS of 0-1 (98.3%).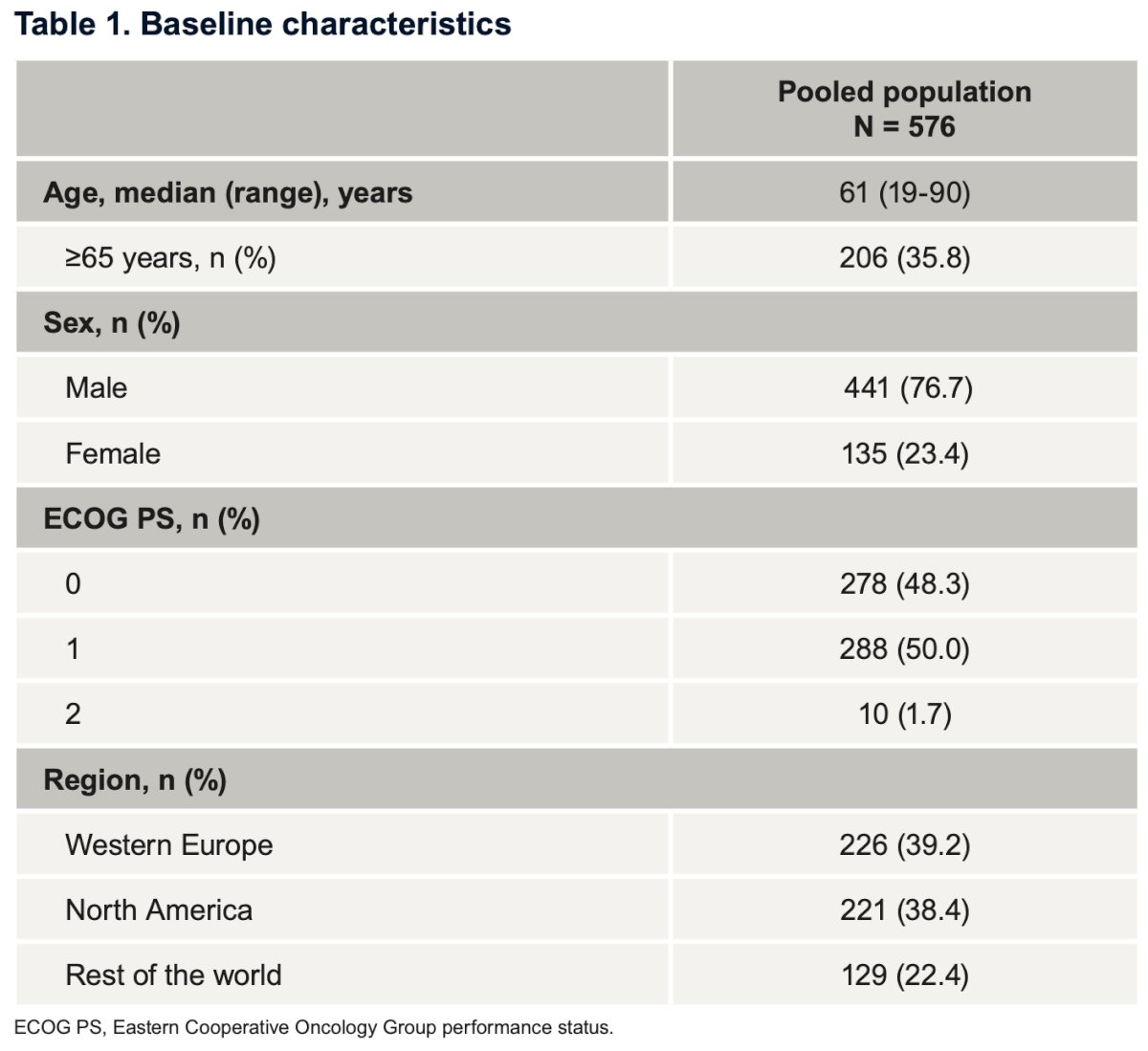
Across the four trials, a total of 526 patients (91.3%) experienced treatment-related adverse events (AEs). Grade 3-5 AEs were reported in 37.7% of patients. However, AEs led to treatment discontinuation in only 37 cases (6.4%) and resulted in death in 19 cases (3.3%).
Anemia and hypoxia were among the most frequent AEs, affecting 84.2% and 16.3% of patients, respectively. Fatigue and nausea were also common, occurring in 42.7% and 24.1% of patients, respectively. The graphic below illustrates any-cause AEs with an incidence of ≥10%.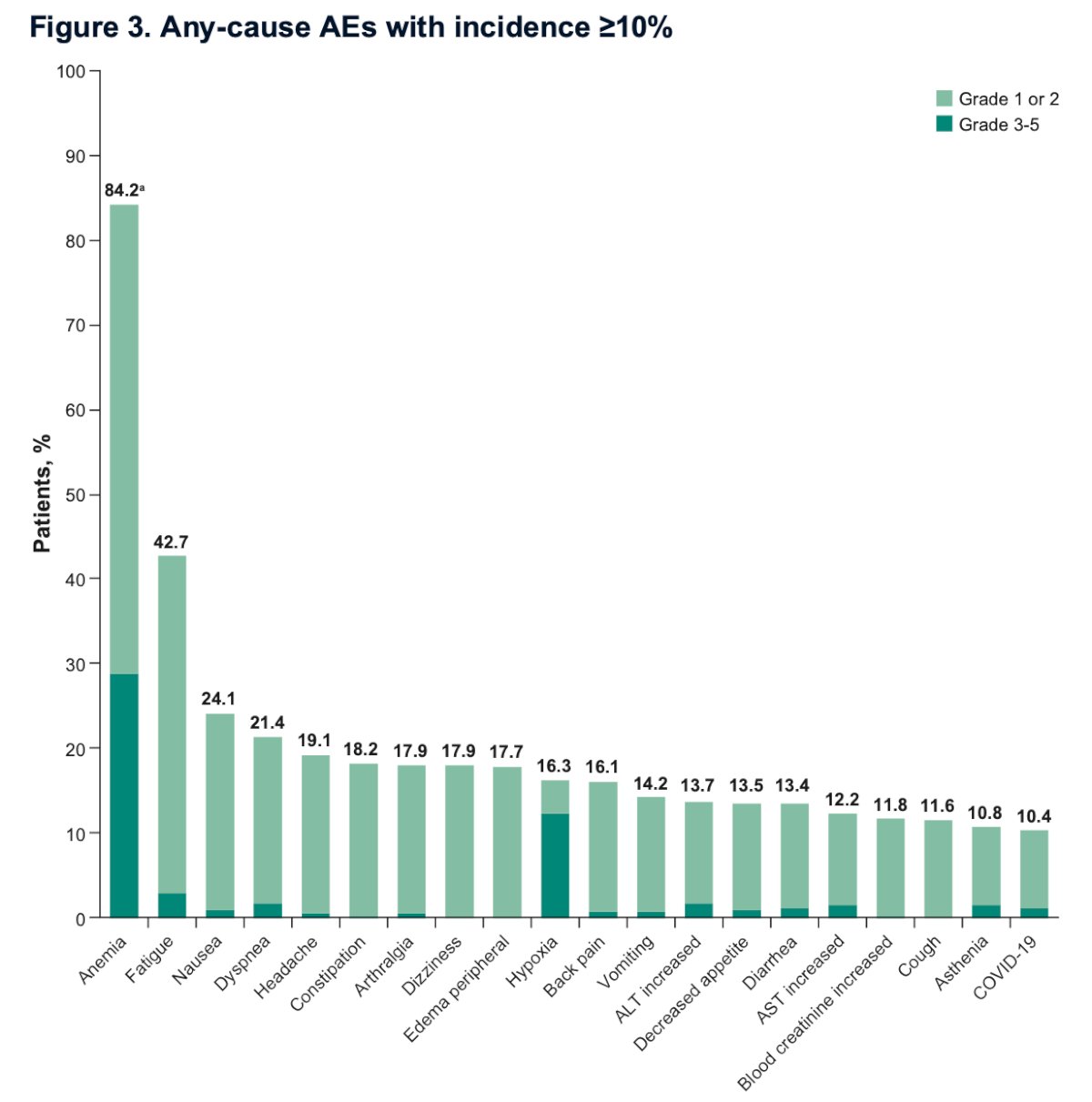
The median time to first onset of AEs occurred within the first three months of treatment. Specifically, the median time to first onset was 29 days for anemia, 31 days for hypoxia, and 42 days for fatigue.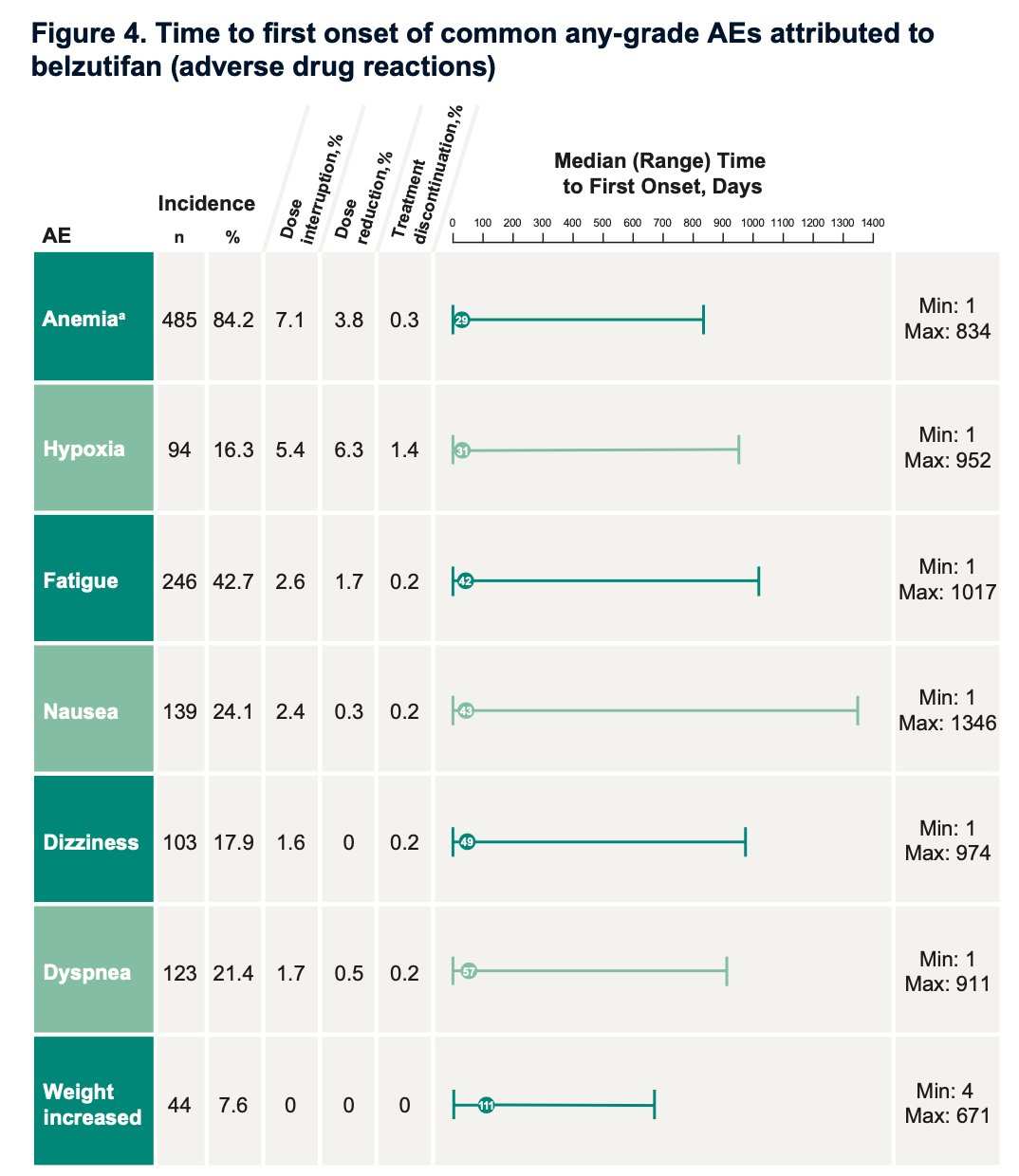
Of the patients who presented with anemia, 22.9% were managed with erythropoiesis-stimulating agents (ESA) only, 17.5% with blood transfusions only, and 12.8% with both ESA and blood transfusions. The median time to onset of ESA use was 85 days, with a median of five injections per patient. The median time to the first blood transfusion was 87 days, with a median of two transfusions per patient.
Patients with hypoxia were treated with supplemental oxygen in 70.2% of cases. The median time to onset of supplemental oxygen therapy was 43 days, with a median duration of 9 days. The median time to resolution of hypoxia was 11 days.
Dr. Jonasch concluded his presentation with the following key messages:
- To date, this is the largest pooled safety dataset for patients who received HIF-2a inhibitor for RCC
- .Belzutifan demonstrated a generally manageable safety profile, with few patients discontinuing treatment due to AEs (6%) in a pooled analysis of 576 patients across four clinical trials.
- Treatment related AEs had a relatively early onset, with a median time to first onset occurring within the first 3 months of treatment.
- Anemia and hypoxia were among the most frequent AEs, which were mostly managed with dose modifications and/or treatments like ESA/blood transfusions for anemia and supplemental oxygen for hypoxia.
- Belzutifan offers a unique mechanism of action compared to other RCC treatments and is the only treatment approved for patients with VHL disease-associated tumors.
Presented by: Eric Jonasch, MD, Professor in the Department of Genitourinary Medical Oncology, Division of Cancer Medicine, at the MD Anderson Cancer Center of The University of Texas.
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, between the 3rd and 6th of December, 2024.
References:- Albiges L, Rini BI, Peltola K, et al. LBA88 Belzutifan versus everolimus in participants (pts) with previously treated advanced clear cell renal cell carcinoma (ccRCC): randomized open-label phase III LITESPARK-005 study. Ann Oncol. 2023;34(suppl 2):S1329-S1330.
- Agarwal N, Brugarolas J, Ghatalia P, George S, Haanen JB, Gurney H, Ravilla R, Van der Veldt A, Beuselinck B, Pokataev I, Suelmann BBM, Tuthill MH, Vaena D, Zagouri F, Wu J, Perini RF, Liu Y, Merchan J, Atkins MB. Randomized phase II dose comparison LITESPARK-013 study of belzutifan in patients with advanced clear cell renal cell carcinoma. Ann Oncol. 2024 Sep 2:S0923-7534(24)03918-8. doi: 10.1016/j.annonc.2024.08.2338. Epub ahead of print. PMID: 39233312..
- Jonasch E, Donskov F, Iliopoulos O, Rathmell WK, Narayan VK, Maughan BL, Oudard S, Else T, Maranchie JK, Welsh SJ, Thamake S, Park EK, Perini RF, Linehan WM, Srinivasan R; MK-6482-004 Investigators. Belzutifan for Renal Cell Carcinoma in von Hippel-Lindau Disease. N Engl J Med. 2021 Nov 25;385(22):2036-2046. doi: 10.1056/NEJMoa2103425. PMID: 34818478; PMCID: PMC9275515.


