(UroToday.com) The 2024 SUO annual meeting included a kidney cancer session, featuring a presentation by Dr. Brian Shuch discussing advances in RCC molecular imaging and theranostics. There are currently many unmet needs in RCC where nuclear medicine can play a role. Currently, we have difficulty characterizing renal masses and identifying distant disease, and we need tools to predict/monitor treatment response. Nuclear medicine modalities are available for many of these in other disease states and some can be exploited for treatment purposes.
With regards to current tools, 99Tc-MIBI identifies cells with high mitochondrial content, such as chromophobe RCC, oncocytoma, hybrid oncocytic/chromophobe tumor, and low grade oncocytic tumor. The specificity of 99Tc-MIBI is ~90-95%, and “hot” cases are ideal for surveillance. However, the limitations include a low sensitivity and relatively low NPV, it does not distinguish from benign disease, and the role is less defined with larger lesions (less benign lesions):
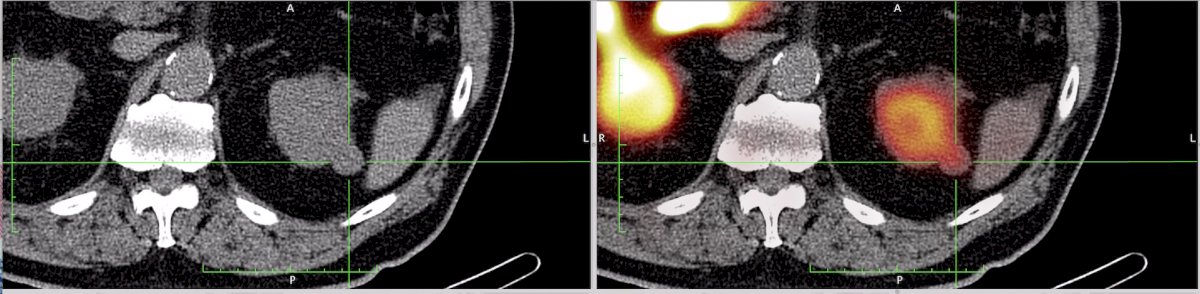
Dr. Shuch then discussed FDG, which enters the cells via glucose transport and is subsequently trapped. The SUV on PET is somewhat equivalent to the metabolic activity, however there is limited use with primary RCC detection. Moreover, there is physiological renal excretion and benign lesions can be metabolically active. FDG PET/CT may be more useful for assessing distant disease, as kidney uptake and excretion are less relevant for distant disease. The initial FDG PET series showed poor sensitivity (60%) but excellent specificity but was in the era prior to PET/CT fusion. A more contemporary 2023 meta-analysis of 11 studies and 1,300 patients suggested a sensitivity of 95% and specificity of 95%.1 FDG avidity may also have a role in prognosis given a biologic basis (ccB phenotype HIF1a/glycolytic phenotype). However, FDG PET/CT is not recommended by current clinical guidelines.
Dr. Shuch then discussed PSMA and the kidney, given that PSMA (aka FOLH1)-folate hydrolase 1 is overexpressed in the kidney proximal tubule. PSMA is also found in the neovasculature of the majority of renal cortical tumors (endothelial cells) and has high expression in 83% of clear cell RCC. However, intense renal uptake likely limits the use as a primary renal tumor imaging modality:
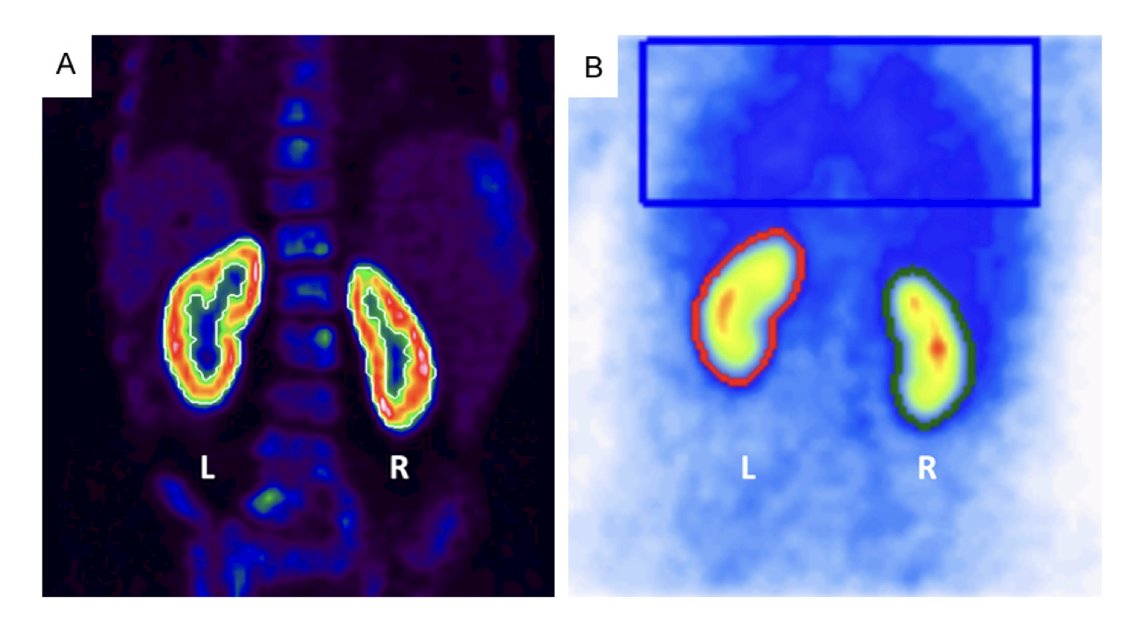
In a small study of 37 patients with RCC enrolled at Johns Hopkins University that underwent PSMA imaging,2 17/21 (81.0%) had metastatic lesions confirmed to have PSMA uptake with a median SUVmax for the metastatic sites of 2.7 (range: 0.9-38.5). Among 14 patients with oligometastatic disease on conventional imaging, 4/14 (28.5%) patients had additional lesions detected:
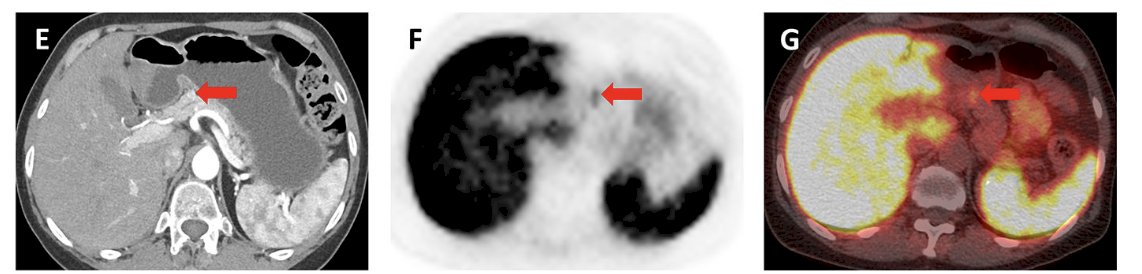
Dr. Shuch notes that this may confirm the suspicion, but low tumor/background ratio limits the identification of new sites.
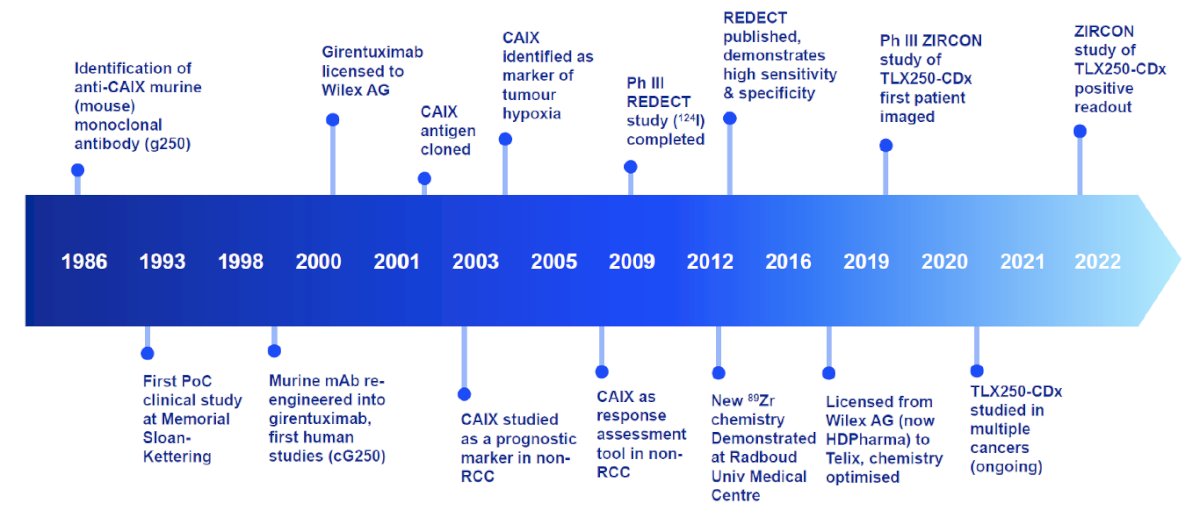
At the cellular level, CAIX plays a role in intracellular pH regulation and prevents intracellular acidosis, thus promoting cell survival. With regards to CAIX and clear cell RCC, the following timeline demonstrates the long odyssey to the clinic:
Girentuximab is a IgG1 kappa light chain chimeric monoclonal antibody that binds with high specificity to CAIX and is internalized. Hepatobiliary excretion allows optimal renal visualization and various types of payloads can be attached for excellent imaging of CAIX. There is extensive safety experience with girentuximab in prior imaging and therapeutic studies, and imaging clear cell RCC has proven feasible with both PET/CT and SPECT/CT using labeled girentuximab. Dating back to 2013, the REDECT study assessed pre-operative 124I-girentuximab for imaging of renal masses, with primary endpoints of sensitivity and specificity (confidence intervals >75%).3 Among 166 patients, any size mass was included (range: 0.2 – 22 cm), although patients with renal masses <7 cm were present in 81.0% of the cohort. However, there was a significant dropout rate (only 86% were evaluable) and there was a low non-clear cell RCC rate, thus the study was underpowered and failed to meet its primary endpoint:
The ZIRCON trial was initially presented ASCO GU 2023 and subsequently published in Lancet Oncology in 2024.4 This was an open-label, multicenter clinical trial including patients with indeterminate renal masses (≤ 7 cm; tumor stage cT1) who were scheduled for partial nephrectomy within 90 days from planned 89Zr-DFO-girentuximab administration. Enrolled patients received a single dose of 89Zr-DFO-girentuximab IV (37 MBq ± 10%; 10 mg girentuximab) on Day 0 and underwent PET/CT imaging on Day 5 (± 2 days) prior to surgery:
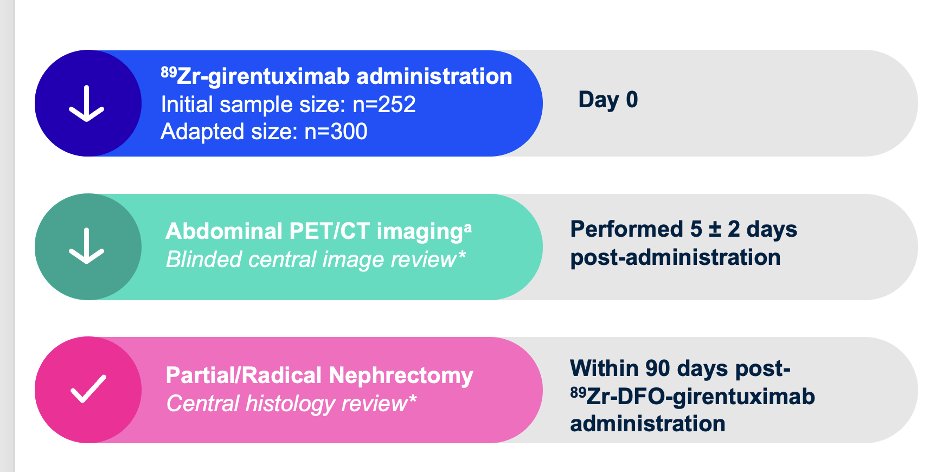
Blinded central histology review determined clear cell RCC status. The co-primary objectives were to evaluate both the sensitivity and specificity of 89Zr-DFO-girentuximab PET/CT imaging in detecting clear cell RCC in patients with indeterminate renal masses, using histology as the standard of truth. Key secondary objectives included sensitivity and specificity of 89Zr-DFO-girentuximab PET/CT imaging in the subgroup of patients with indeterminate renal masses ≤ 4 cm (cT1a). Other secondary objectives included positive and negative predictive values, safety, and tolerability.
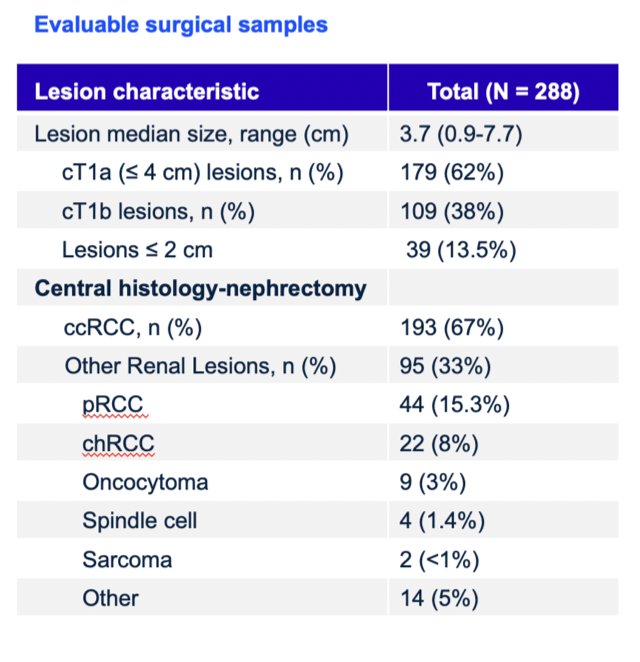
There were 332 patients enrolled between August 2019 and August 2022 among 36 sites in 9 countries that received 89Zr-DFO-girentuximab. The median age was 62 years (range: 27-87), and 71.3% of patients were male. Of 288 patients with central histopathology of surgical samples, 193 (67%) had clear cell RCC, and 179 (62%) had cT1a disease:
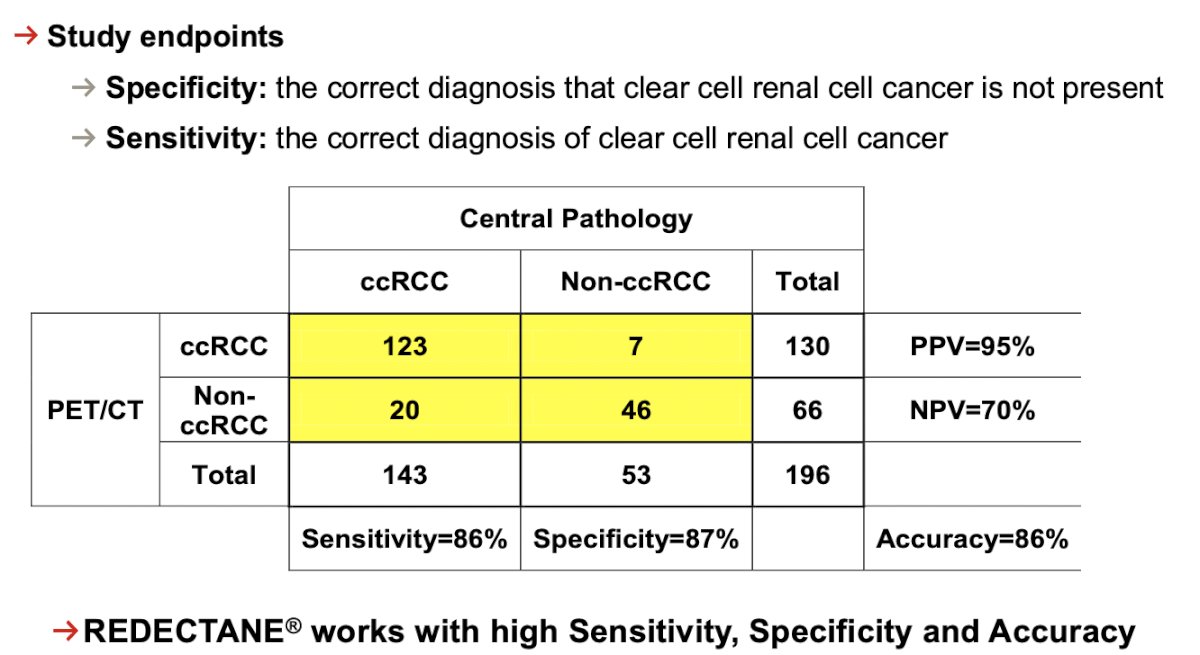
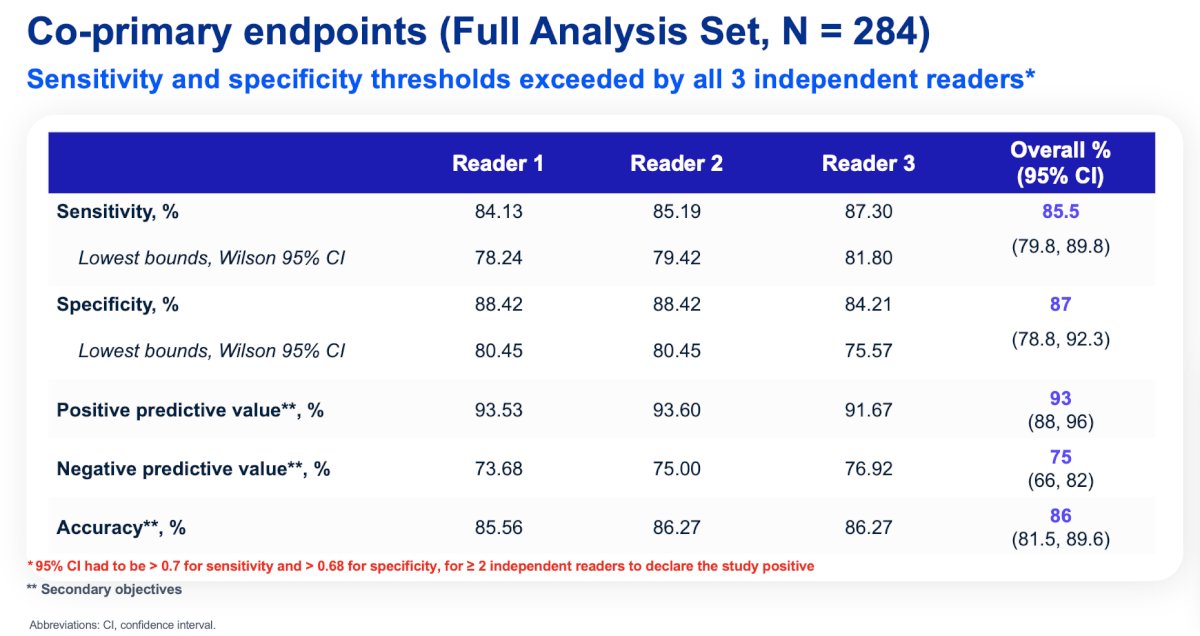
Of 284 evaluable patients included in primary analysis, the average across all 3 readers for sensitivity and specificity was 86% [95% CI 80%, 90%] and 87% [95% CI 79%, 92%], respectively, for co-primary endpoints, and 85% [95% CI 77%, 91%] and 90% [95% CI 79%, 95%], respectively, for key secondary endpoints. For all readers, the lower boundaries of 95% CI for co-primary and key secondary endpoints were > 75%. For all evaluable patients, positive and negative predictive values were ≥ 91.7% and ≥ 73.7%, respectively:
Very few adverse events were considered possible or related to 89Zr-DFO-girentuximab. Most adverse events were mild, with only 18 patients (6%) having a Grade >= 3 treatment emergent adverse event. The adverse event pattern was consistent with post-surgical complications related to the nephrectomy, and no unexpected safety signals were observed.
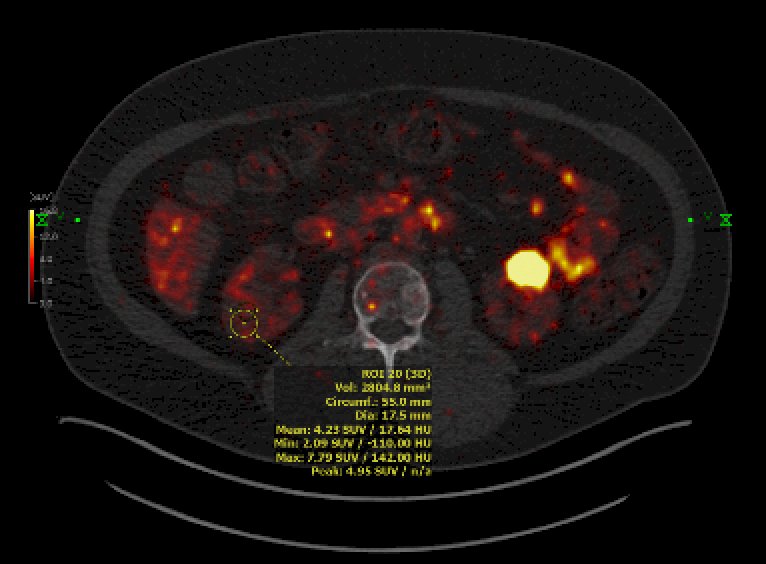
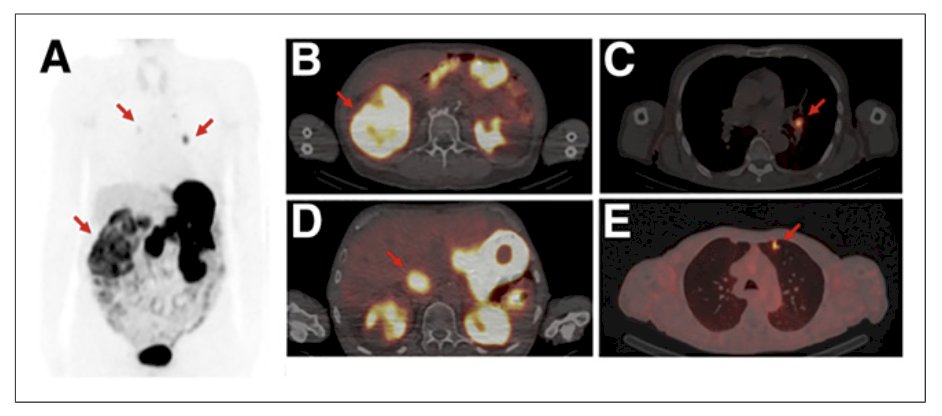
Dr. Shuch then discussed the IMPACT-RCC trial5 which evaluated the lesion detection of baseline contrast-enhanced CT, 89Zr-DFO-girentuximab-PET/CT, and 18F-FDG-PET/CT in detecting clear cell RCC lesions in patients with a good or intermediate prognosis metastatic clear cell RCC according to IMDC risk model. Among 42 patients, 449 lesions were detected by ≥1 modality (median per patient: 7) of which 42% were in the lung, 22% in lymph nodes, and 10% in bone. Combined 89Zr-DFO-girentuximab-PET/CT and CT detected more lesions than CT alone: 91% (95% CI: 87-94) versus 56% (95% CI: 50-62, p = 0.001), respectively, and more than CT and 18F-FDG-PET/CT combined (84% (95% CI:79-88, p < 0.005):
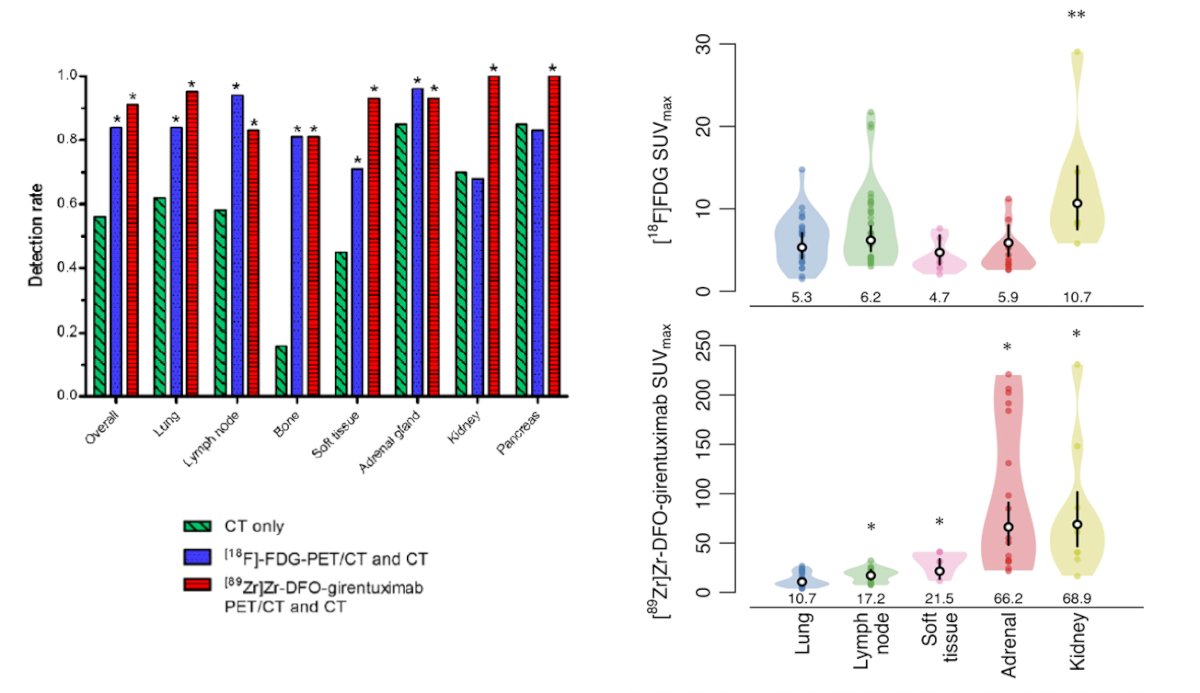
Notably, the SUV was well above background with 89Zr-DFO-girentuximab imaging (SUV 10-250).
Dr. Shuch then discussed the CA NINE trial which is a prospective comparison of contrast-enhance CT against 89Zr-TLX250 PET/CT for the imaging-based detection of recurrence clear cell RCC after surgery:
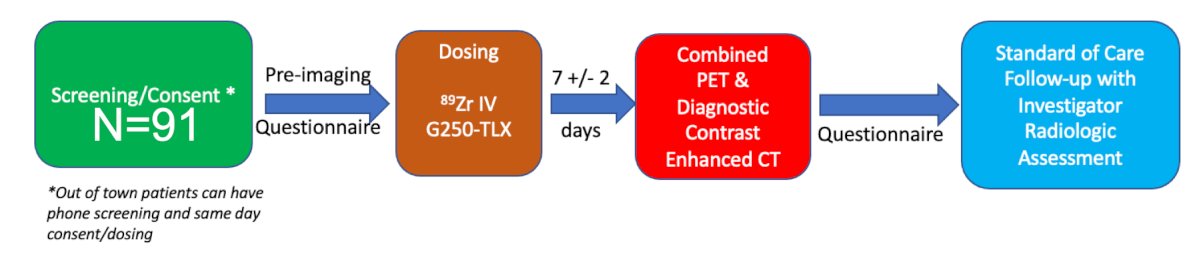
In this ongoing trial, patients have their first post-operative imaging 4-16 weeks after surgery at UCLA, and the adjuvant therapy plan does not impact participation. The primary endpoint is lesion detection (PET/CT versus CT) and the secondary endpoint is PPV for those with lesion validation, recurrence free survival, and the impact on management. The trial currently has enrolled six patients and Dr. Shuch is encouraging providers to reach out for their patients to be enrolled in the clinical trial at UCLA.
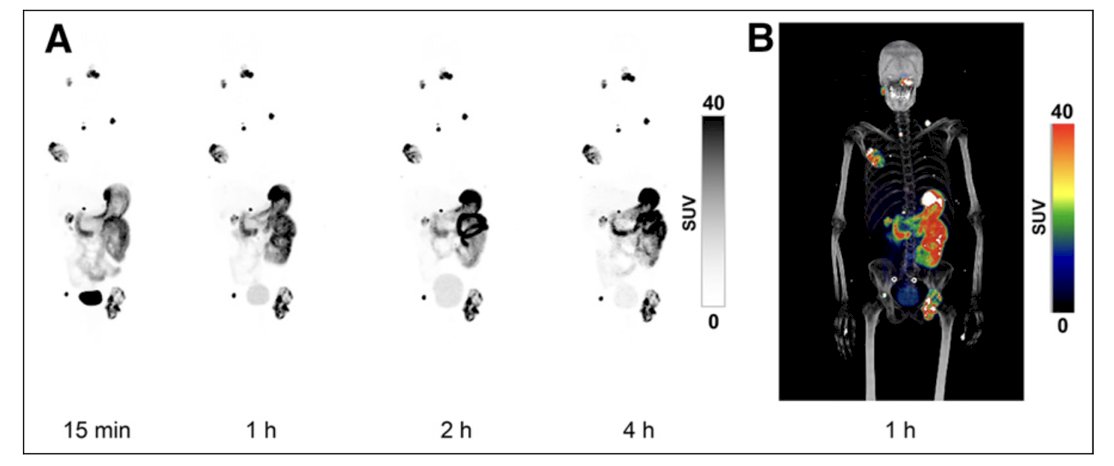
Dr. Shuch then discussed several small molecules for imaging starting with DPI-4452 PET/CT imaging. Three patients with metastatic clear cell RCC treated with 2+ prior lines of therapy were injected with 68Ga-DPI-4452, a CAIX-binding radiolabeled peptide with a 177Lu-DPI-4452 theranostic pair.6 Patients then had whole body PET/CT at 15 minutes, 1 hour, 2 hours, and 4 hours. There were no adverse events and patients had the expected biliary/gastric background uptake (but was very high):
Second, PHC-102 PET/CT (an acetazolamide analogue) imaging was assessed in 5 patients with localized or metastatic clear cell RCC.7 Whole body PET/CT was obtained at 30 minutes, 2 hours, and 6 hours after injection. There were no adverse events, the background biliary/gastric uptake was expected, and renal clearance and high renal/urinary uptake were noted:
With regards to external delivery of radiation in kidney cancer, for primary kidney cancer treatment, there are dose limitations by surrounding structures (ie. duodenum, colon, etc), the tumor still enhances and is viable after treatment (though has “local control”), and there is moderate renal toxicity. For distant sites, SBRT appears to impact the metastatic RCC disease course, although collateral damage to adjacent tissue may limit dosing and the number of treatments. As such, are there alternative ways to safely and effectively deliver ionizing radiation? Local theranostics include microsphere radionuclide delivery. Specifically, trans-arterial chemotherapy (TACE) is modified for radionuclide delivery in which synthetic microparticles (resin or glass) are bound to radioactive elements. Y90 (a beta emitter) is delivered into the tumor arterial vasculature and provides very high radiation doses (100-400 Gy). The RENEGADE trial is an open-label, prospective, multi-center, phase 1/2 safety trial of theraspheres, of which Dr. Shuch is the urology PI. Patients with cT1/T2a renal masses will undergo Y90 glass microspheres with a planned dose of 400 Gy and a primary objective of assessing safety and toxicity:
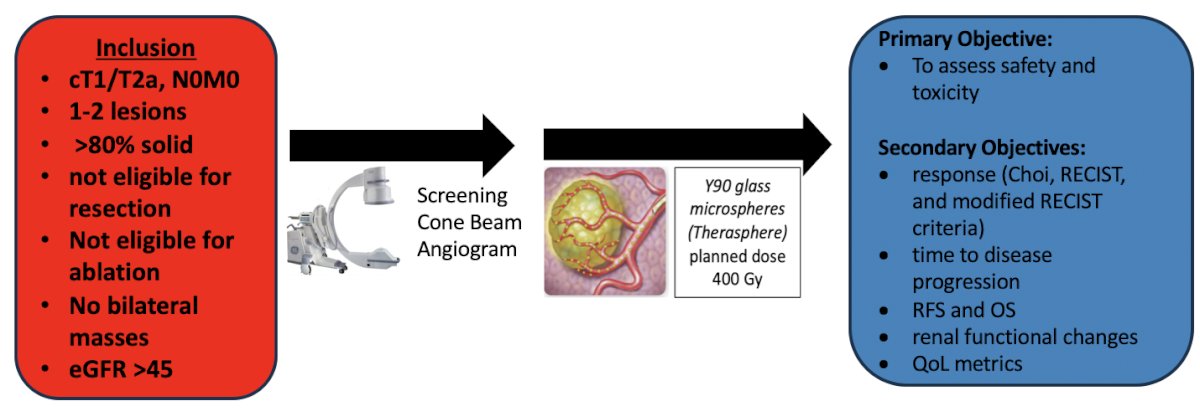
This trial opened December 1, 2024 at UCLA, with plans to open the trial at Cedars Sinai, Loyola, and the University of Washington.
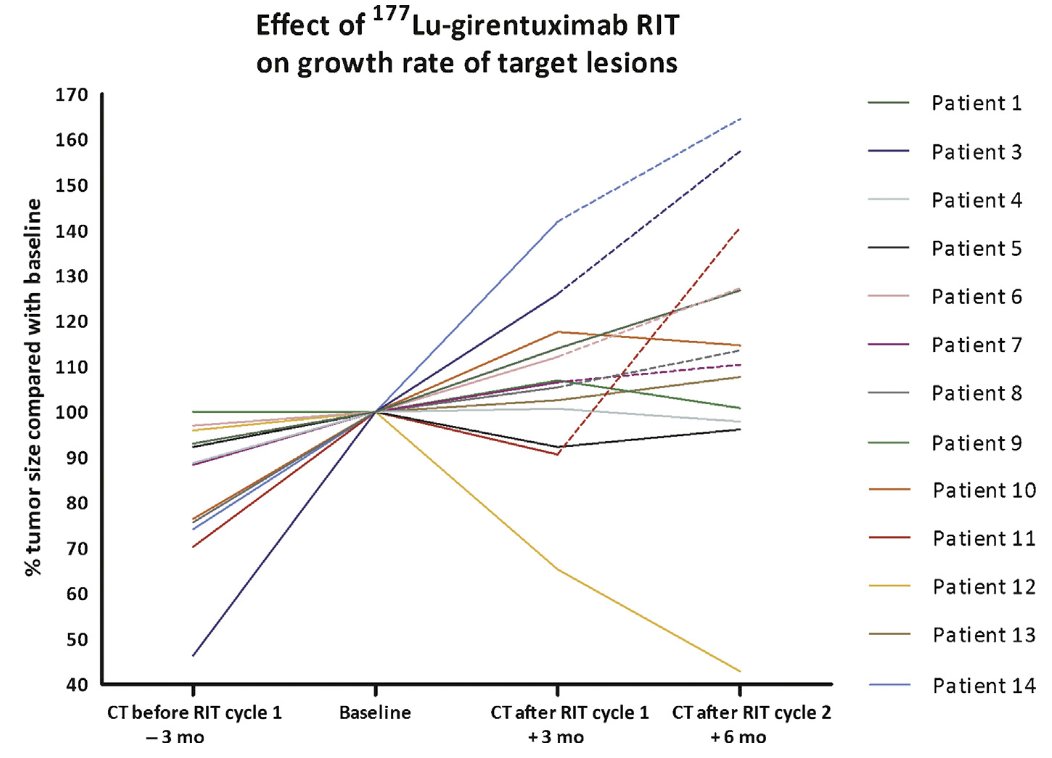
Systemic theranostics is similar to PSMA and dotatate approaches in that once you can see a lesion, we can quickly expand into systemic treatment. CAIX is highly selective for clear cell RCC and targeting with theranostics with an alpha or beta emitter is the next frontier. Furthermore, is there potential synergy with selective DNA damage agents? With immunotherapy? A phase 2 Dutch trial from 2016 assessed the utility of 177Lu-girentuximab treatment with progressive M1 clear cell RCC with one dose of treatment, but an option for two doses.8 Overall, 9/14 patients had stable disease per RECIST, with some grade 3/4 myelotoxicity:
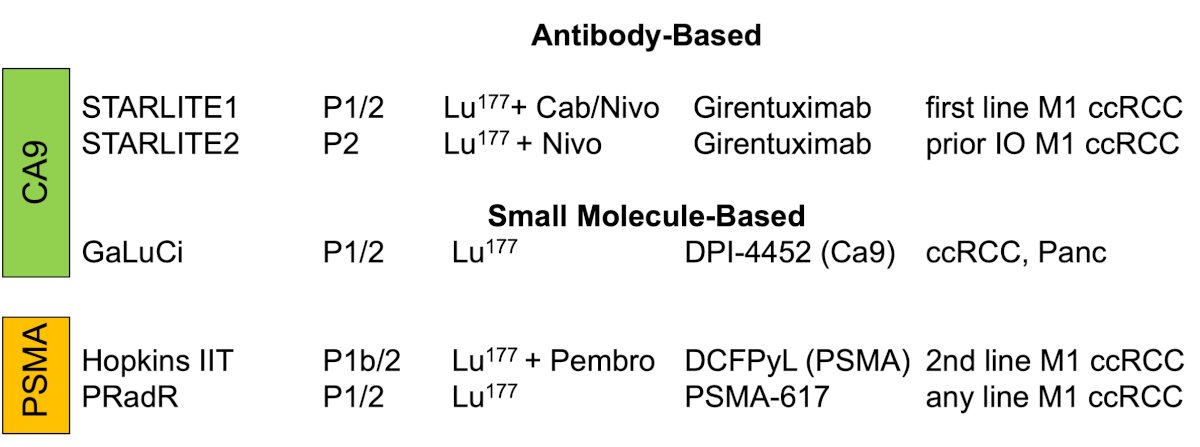
Currently, there are several theranostic trials ongoing for clear cell RCC:
Dr. Shuch concluded his presentation by discussing advances in RCC molecular imaging and theranostics with the following take home messages:
- There are many unmet imaging needs where nuclear medicine imaging can play an important role
- Current tools like SPECT/CT and FDG/PSMA PET/CT have limitations
- The CAIX PET/CT revolution is coming to RCC:
- 89Zr-DFO-girentuximab can identify primary clear cell RCC
- Distant disease can be detected with 89Zr-DFO-girentuximab
- Small molecules are on the horizon
- Theranostics is a promising avenue for treatment alone or in combination, with both local and systemic delivery trials ongoing
Presented by: Brian Shuch, MD, University of California Los Angeles, Los Angeles, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, between the 3rd and 6th of December, 2024.
References:
- Fan L, Xu Y, Zhao J, et al. The diagnostic performance of 18F-FDG PET/CT in recurrent renal cell carcinoma: A systematic review and meta-analysis. Clin Transl Imaging 2023(11):199-208.
- Meyer AR, Carducci MA, Denmeade SR, et al. Improved identification of patients with oligometastatic clear cell renal cell carcinoma with PSMA-targeted 18F-DCFPyL PET/CT. Ann Nucl Med. 2019 Aug;33(8):617-623.
- Divgi CR, Uzzo RG, Gatsonis C, et al. Positron emission tomography/computed tomography identification of clear cell renal cell carcinoma: Results from the REDECT trial. J Clin Oncol. 2013 Jan 10;31(2):187-194.
- Shuch B, Pantuck AJ, Bernhard JC, et al. [89Zr]Zr-girentuximab for PET-CT imaging of clear-cell renal cell carcinoma: A prospective, open-label, multicentre, phase 3 trial. Lancet Oncol. 2024 Oct;25(10):1277-1287.
- Verhoeff SR, van Es SC, Boon E, et al. Lesion detection by [89Zr]Zr-DFO-girentuximab and [18F]FDG-PET/CT in patients with newly diagnosed metastatic renal cell carcinoma. Eur J Nucl Med Mol Imaging. 2019 Aug;46(9):1931-1939.
- Hofman MS, Tran B, Feldman DR, et al. First-in-Human Safety, Imaging, and Dosimetry of a Carbonic Anhydrase IX–Targeting Peptide, [68Ga]Ga-DPI-4452, in Patients with Clear Cell Renal Cell Carcinoma. J Nucl Med. 2024 Feb 22;65(5):740-743.
- Kulterer OC, Pfaff S, Wadsak W, et al. A Microdosing Study with 99mTc-PHC-102 for the SPECT/CT Imaging of Primary and Metastatic Lesions in Renal Cell Carcinoma Patients. J Nucl Med. 2021 Mar;62(3):360-365.
- Muselaers CJH, Boers-Sonderen MJ, van Oostenbrugge TJ, et al. Phase 2 Study of Lutetium 177–Labeled Anti–Carbonic Anhydrase IX Monoclonal Antibody Girentuximab in Patients with Advanced Renal Cell Carcinoma. Eur Urol. 2016 May;69(5):767-770.


