(UroToday.com) The 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, between December 3 and December 6, 2024, was host to the Kidney Cancer Session II. Dr Mark Ball discussed Biomarkers and Clinical Predictors in Renal Cell Carcinoma (RCC).
Dr. Ball began by emphasizing the importance of understanding key definitions before discussing biomarkers and predictors for RCC. He clarified that there are two types of biomarkers: predictive and prognostic. Predictive biomarkers are used to determine whether a patient will respond to a specific therapy, while prognostic biomarkers predict the likelihood of an event, such as recurrence or metastasis, occurring. In RCC, biomarkers can be applied in both localized or locally advanced and advanced disease settings. These biomarkers can be derived either from the tumor itself or from the host.
In addition to biomarkers, there are other prediction tools that can assist in estimating the probability of malignancy and high-grade RCC in solid tumors, predicting oncological outcomes postoperatively, forecasting progression after nephrectomy, and even determining postoperative cancer-specific survival. A valuable resource for these tools is the website Cancer Nomograms, which provides a list of nomograms for various urological malignancies, including RCC at all stages of the disease.
We have several clinical predictors that can guide decision-making in RCC. These include the Leibovich score (which predicts metastasis-free survival), the SSIGN score (which predicts cancer-specific survival), and the ASSURE nomogram (which predicts overall survival, disease-free survival, and early disease progression). These tools should be used by clinicians to help predict oncological outcomes, inform patients, and improve treatment decision-making.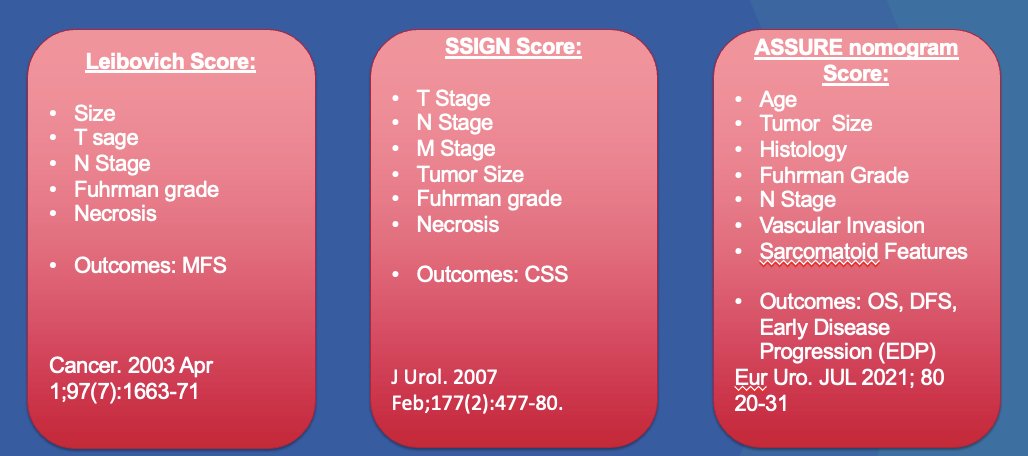
Another clinical predictor we can use is the growth rate of different tumor subtypes. Two studies1,2 found that tumor growth rates varied significantly across genetic and tumor subtypes. In a study led by Dr. Ball, rapid growth of BAP1-deficient tumors indicated that these patients should be managed with caution. The faster growth observed in younger patients may warrant more frequent imaging, whereas the slower growth of other tumor subtypes could support extended surveillance beyond annual imaging in some cases.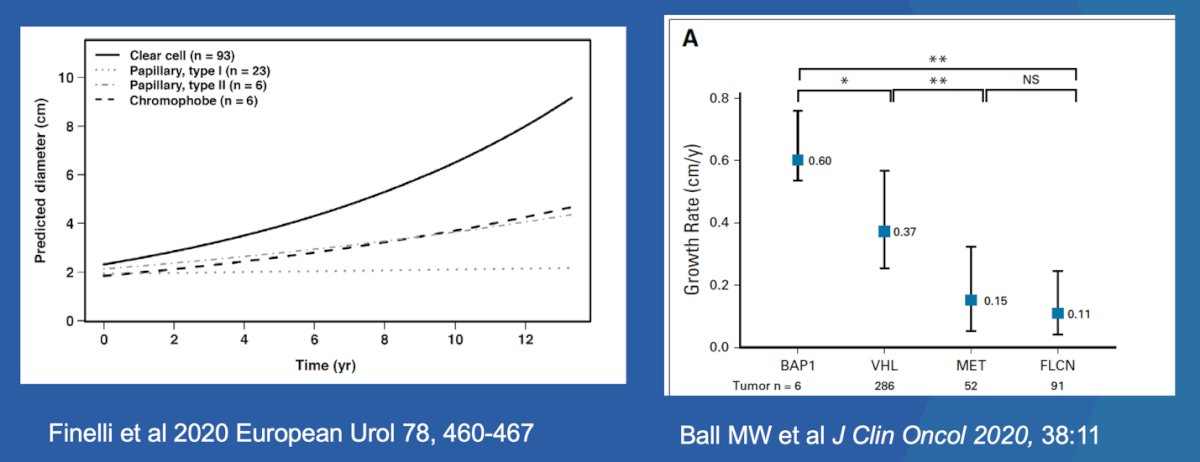
Dr. Ball mentioned that in a recent poll on Twitter, he asked urologic oncologists at SUO24 how many had frequently used a predictive cancer nomogram for a patient with localized RCC in the last 90 days. Astonishingly, 80% responded that they had never used one, highlighting that despite having access to these tools and clinical predictors, they are not being widely utilized in clinical practice.
In the metastatic setting, clinical biomarkers are more commonly used. For example, the IMDC risk classification system utilizes various clinical variables and laboratory results, such as hemoglobin levels, calcium, platelet count, and neutrophil counts, to risk-stratify patients into three categories: favorable, intermediate, and poor risk.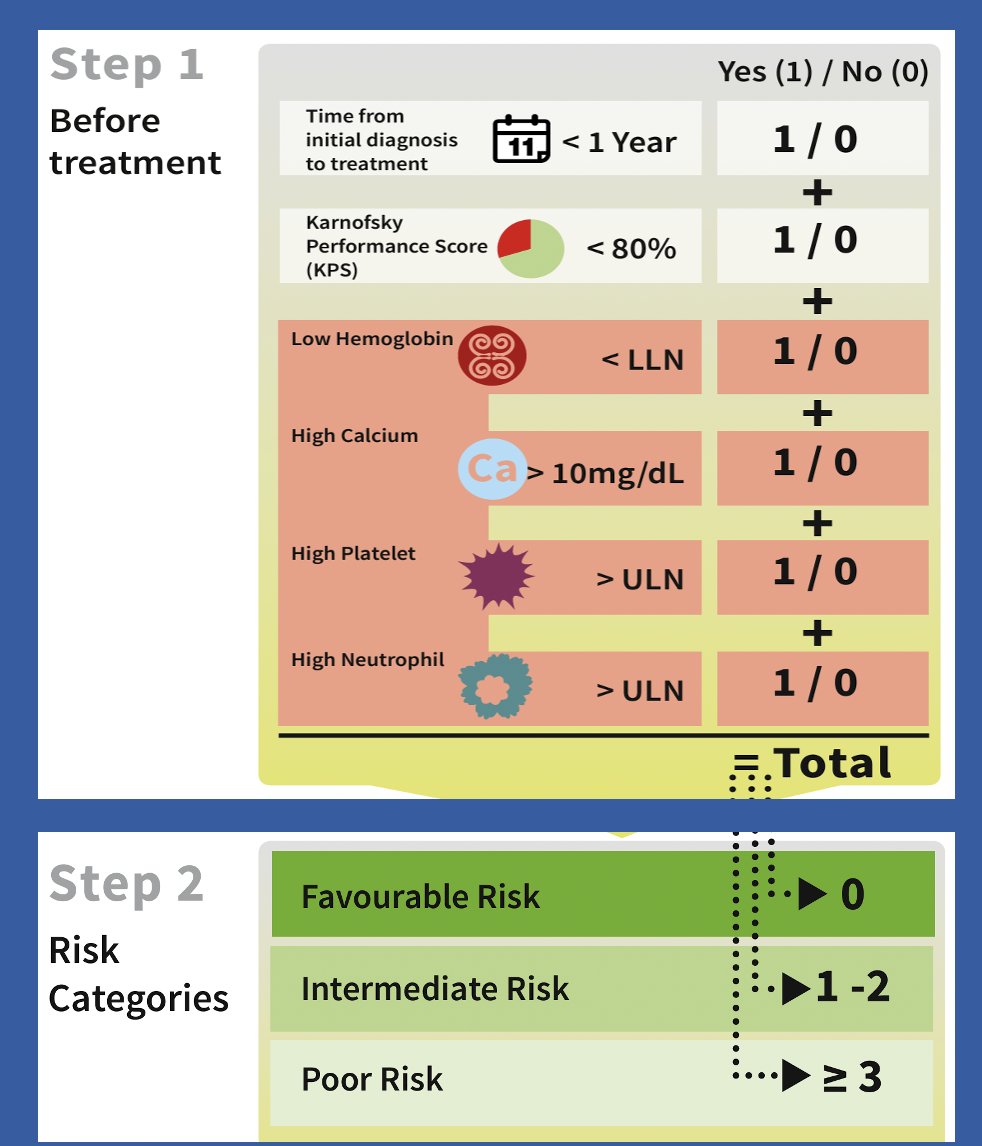
These risk categories have been shown to significantly and efficiently predict overall survival among the three subgroups and are recommended for guiding first-line therapy decisions in mRCC.
A modern clinical or radiological predictor could be [89Zr]Zr-girentuximab PET/CT. In the ZIRCON study, this imaging modality demonstrated a sensitivity of 85.5% and specificity of 87.0% for clear cell RCC.3 If used correctly, it could help inform clinical decision-making in the future and potentially predict response to treatment.![A modern clinical or radiological predictor could be [89Zr]Zr-girentuximab PET/CT. In the ZIRCON study, this imaging modality demonstrated a sensitivity of 85.5% and specificity of 87.0% for clear cell RCC. (3) If used correctly, it could help inform clinical decision-making in the future and potentially predict response to treatment.](/images/com-doc-importer/186-suo-2024/suo-2024-biomarkers-and-clinical-predictors-in-rcc/image-5.jpg)
Dr. Ball pivoted to the biomarker space, noting that the study of biomarkers in RCC is gaining significant attention. There are multiple types of biomarkers we can use for detecting and predicting clinical outcomes in RCC, including ctDNA, microbiome analysis, CD8+ tracers, KIM-1, and basic histology subtype analysis.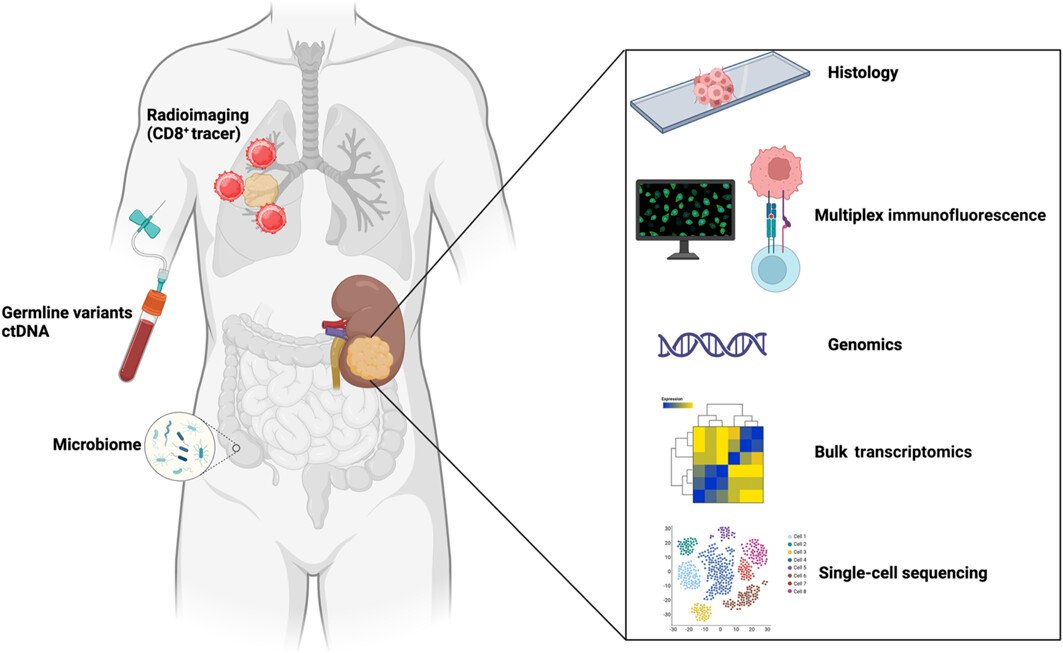
ctDNA has been studied in localized disease preoperatively in a cohort of patients with renal masses suspicious for RCC who underwent either partial or radical nephrectomy. In this study, preoperative ctDNA was detectable in 61% of patients with nonmetastatic RCC and was associated with higher stage, venous invasion, larger tumor size, and higher WHO ISUP grade. While there is still work to be done to refine this biomarker, it remains promising.4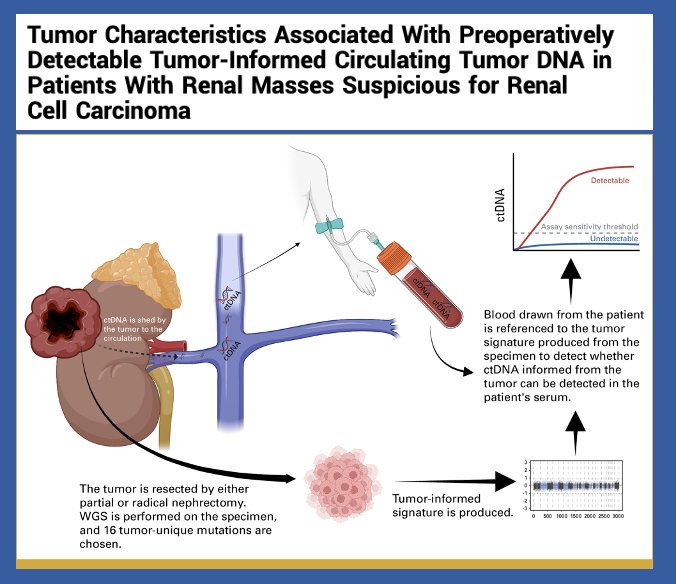
Moreover, levels of CD8+ tumor-infiltrating cells (TIC) expressing PD-1 but not TIM-3 and LAG-3 (IF) have been assessed as potential biomarkers of response to anti-PD-1 therapy.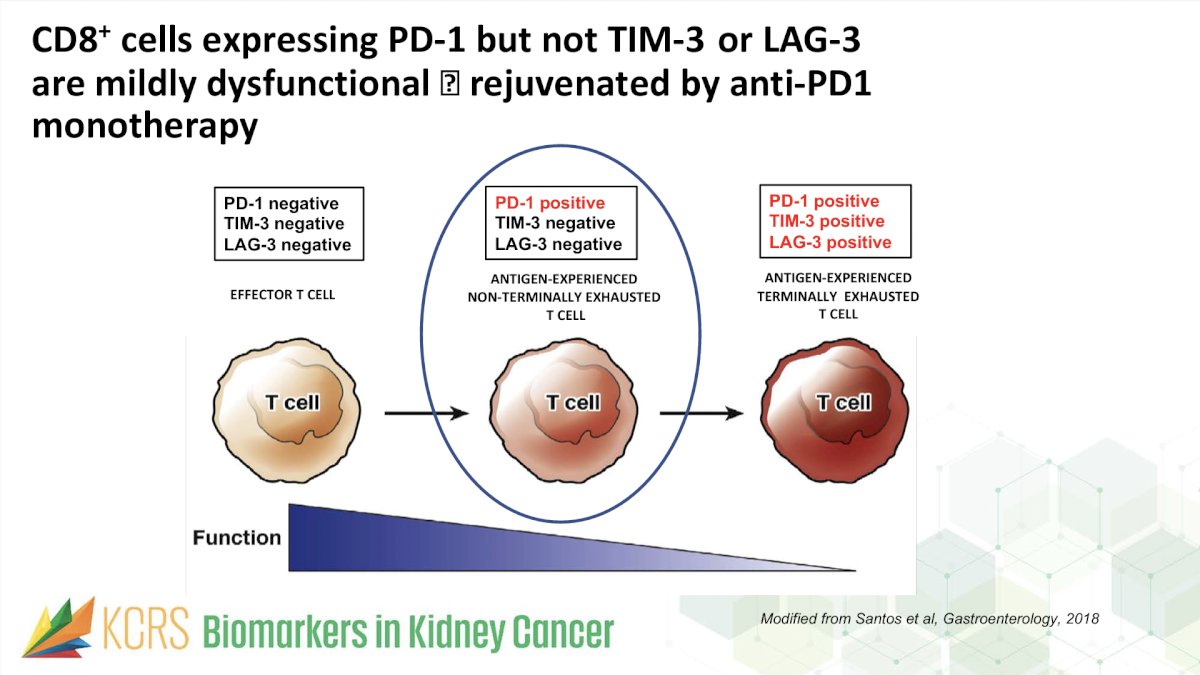
In a study of patients with RCC treated with Nivolumab, high-IF biomarker density had higher ORR (45.8% vs. 19.6%) and longer median PFS (9.6 vs. 3.7 months).5 This also been observed in other three independent cohorts of patients treated with Anti-PD1 therapy.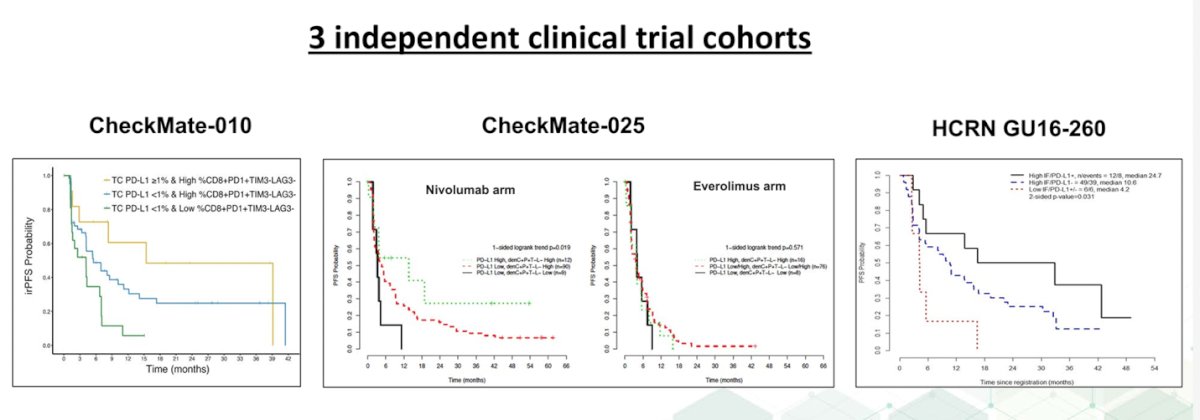
Gene signatures can also potentially serve as predictive biomarkers. In a study led by Motzer, integrated multi-omics analyses were used to identify robust molecular subtypes in 823 tumors from patients with advanced RCC who were part of the IMmotion 151 trial. This study classified patients into 7 clusters as illustrared below. This approach could help in refining personalized treatment strategies based on molecular characteristics of the tumor.6
.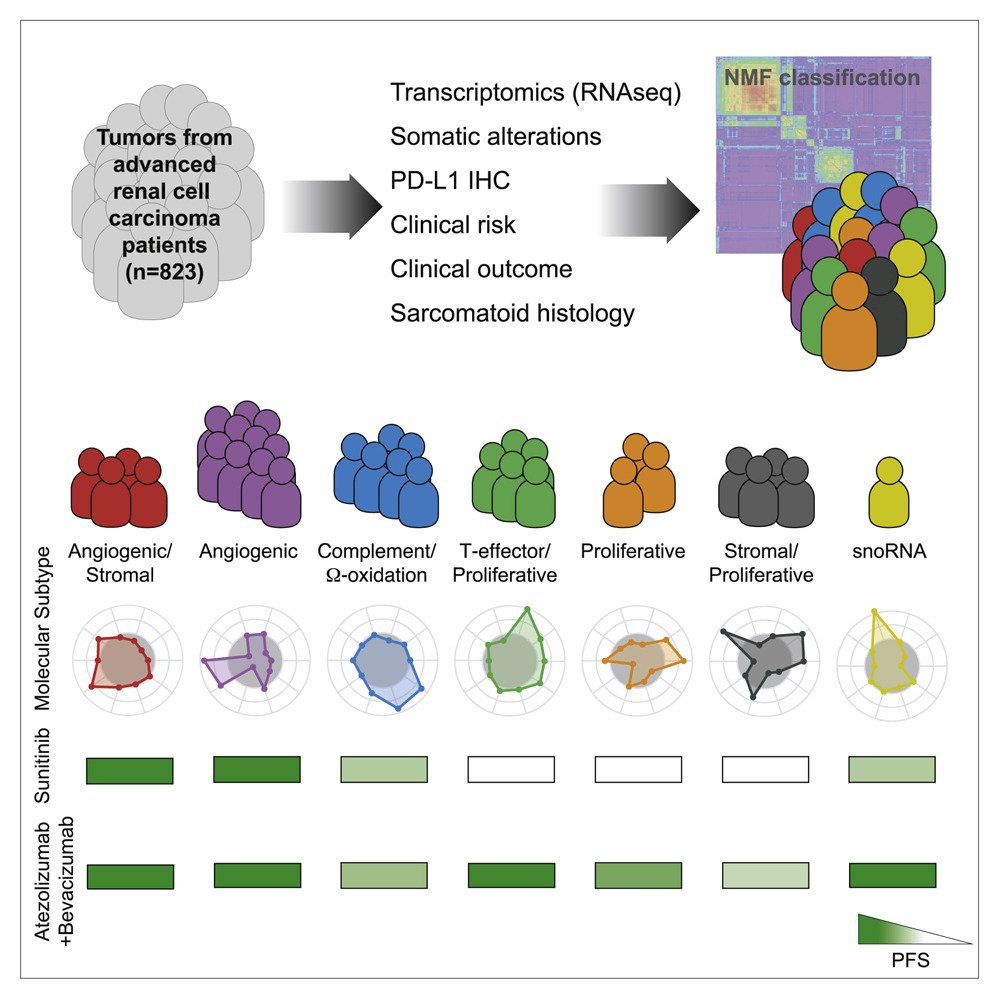
Notably the investigators found that the Cluster’s help to predict response to Sunitinib, Atezolizumab + bevacizumab and could potentially help to guide treatment in the future.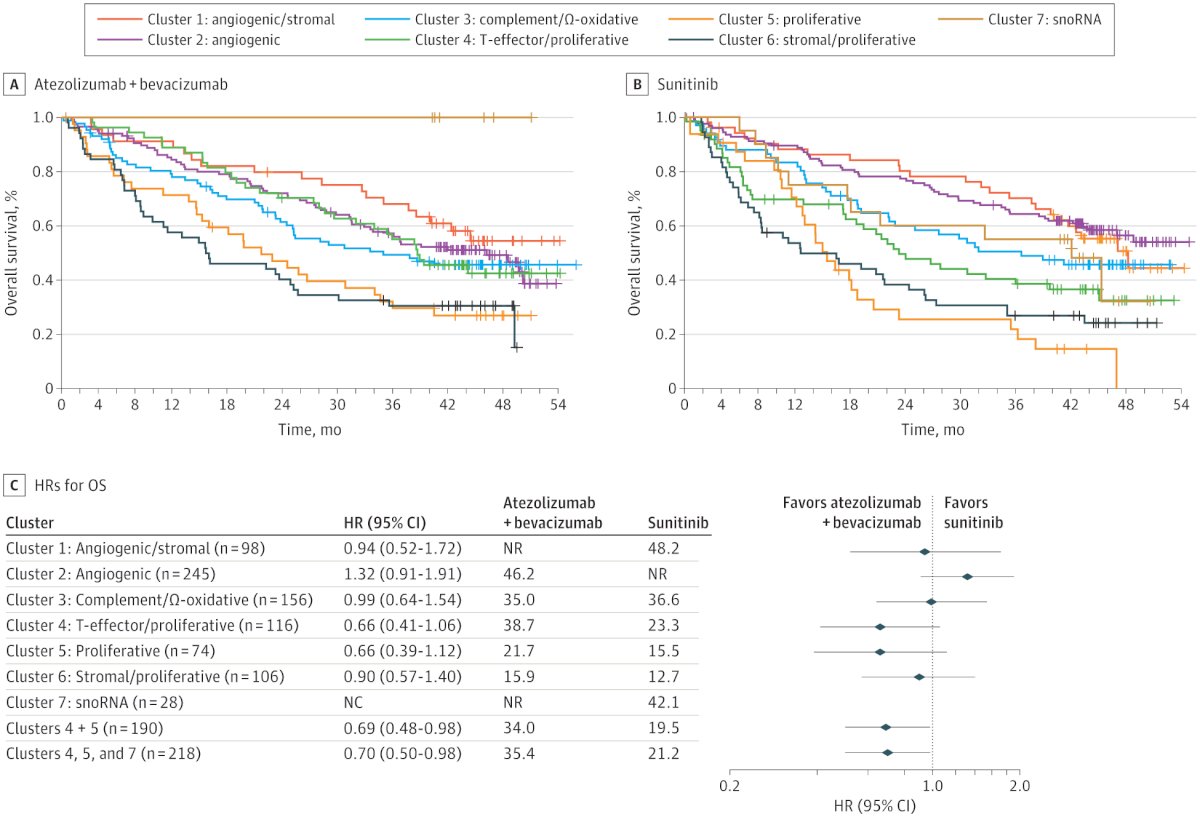
Similarly, gene signatures using 26 genes in the JAVELIN Renal 101 study have also been described, these signatures have also shown potential to predict response to Avelumab + Axitinib but not to sunitinib as shown in the figure below.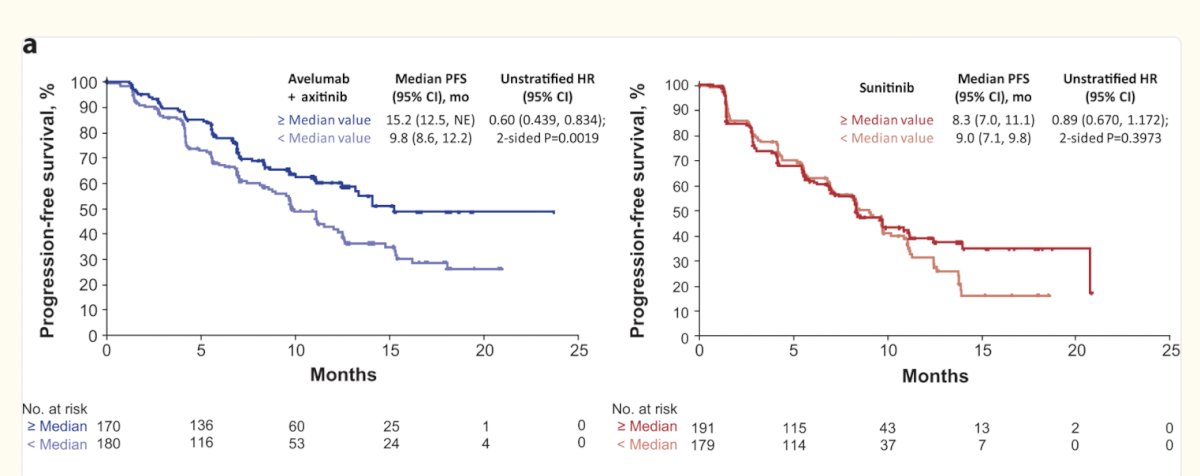
Dr Ball concluded his presentation with the following key messages:
- There are many clinical predictors in localized RCC, however, clinical uptake may be limited
- Biomarkers in localized RCC are emerging; molecular imaging may obviate the need to biopsy in some cases.
- Biomarkers in mRCC including gene signatures are promising, but not quite ready for prime time yet.
Presented by: Mark Ball, MD, FACS, Associate Program Director, Urologic Oncology Fellowship at National Cancer Institute (NCI), Bethesda, Maryland, United States.
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, between the 3rd and 6th of December, 2024.
References:- Finelli A, Cheung DC, Al-Matar A, Evans AJ, Morash CG, Pautler SE, Siemens DR, Tanguay S, Rendon RA, Gleave ME, Drachenberg DE, Chin JL, Fleshner NE, Haider MA, Kachura JR, Sykes J, Jewett MAS. Small Renal Mass Surveillance: Histology-specific Growth Rates in a Biopsy-characterized Cohort. Eur Urol. 2020 Sep;78(3):460-467. doi: 10.1016/j.eururo.2020.06.053. Epub 2020 Jul 14. PMID: 32680677.
- Ball MW, An JY, Gomella PT, Gautam R, Ricketts CJ, Vocke CD, Schmidt LS, Merino MJ, Srinivasan R, Malayeri AA, Metwalli AR, Linehan WM. Growth Rates of Genetically Defined Renal Tumors: Implications for Active Surveillance and Intervention. J Clin Oncol. 2020 Apr 10;38(11):1146-1153. doi: 10.1200/JCO.19.02263. Epub 2020 Feb 21. Erratum in: J Clin Oncol. 2021 Apr 10;39(11):1309. doi: 10.1200/JCO.21.00642. PMID: 32083993; PMCID: PMC7145590.
- Shuch B, Pantuck AJ, Bernhard JC, Morris MA, Master V, Scott AM, van Praet C, Bailly C, Önal B, Aksoy T, Merkx R, Schuster DM, Lee ST, Pandit-Taskar N, Fan AC, Allman P, Schmidt K, Tauchmanova L, Wheatcroft M, Behrenbruch C, Hayward CRW, Mulders P. [89Zr]Zr-girentuximab for PET-CT imaging of clear-cell renal cell carcinoma: a prospective, open-label, multicentre, phase 3 trial. Lancet Oncol. 2024 Oct;25(10):1277-1287. doi: 10.1016/S1470-2045(24)00402-9. Epub 2024 Sep 10. PMID: 39270701.
- Ben-David R, Alerasool P, Kalola H, Tillu N, Almoflihi M, Tsao CK, Galsky MD, Sfakianos JP, Wiklund P, Waingankar N, Mehrazin R. Tumor Characteristics Associated With Preoperatively Detectable Tumor-Informed Circulating Tumor DNA in Patients With Renal Masses Suspicious for Renal Cell Carcinoma. JCO Precis Oncol. 2024 Sep;8:e2400281. doi: 10.1200/PO.24.00281. Epub 2024 Sep 30. Erratum in: JCO Precis Oncol. 2024 Nov;8:e2400733. doi: 10.1200/PO-24-00733. PMID: 39348609.
- Ficial M, Jegede OA, Sant'Angelo M, Hou Y, Flaifel A, Pignon JC, Braun DA, Wind-Rotolo M, Sticco-Ivins MA, Catalano PJ, Freeman GJ, Sharpe AH, Hodi FS, Motzer RJ, Wu CJ, Atkins MB, McDermott DF, Shukla SA, Choueiri TK, Signoretti S. Expression of T-Cell Exhaustion Molecules and Human Endogenous Retroviruses as Predictive Biomarkers for Response to Nivolumab in Metastatic Clear Cell Renal Cell Carcinoma. Clin Cancer Res. 2021 Mar 1;27(5):1371-1380. doi: 10.1158/1078-0432.CCR-20-3084. Epub 2020 Nov 20. PMID: 33219016; PMCID: PMC8443005.
- Motzer RJ, Banchereau R, Hamidi H, Powles T, McDermott D, Atkins MB, Escudier B, Liu LF, Leng N, Abbas AR, Fan J, Koeppen H, Lin J, Carroll S, Hashimoto K, Mariathasan S, Green M, Tayama D, Hegde PS, Schiff C, Huseni MA, Rini B. Molecular Subsets in Renal Cancer Determine Outcome to Checkpoint and Angiogenesis Blockade. Cancer Cell. 2020 Dec 14;38(6):803-817.e4. doi: 10.1016/j.ccell.2020.10.011. Epub 2020 Nov 5. PMID: 33157048; PMCID: PMC8436590.


