(UroToday.com) The 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX between December 3rd – 6th, 2024 was host to a prostate cancer poster session. Dr. A. Oliver Sartor presented the results of a phase III study of 177Lu-TLX591 plus standard-of-care (SOC) versus SOC alone in patients with metastatic castrate-resistant prostate cancer (mCRPC; PROSTACT GLOBAL).
Dr. Scott Tagawa presented ProstACT GLOBAL, a phase III study of best SOC with or without 177Lu-DOTA-rosopatamab (TLX591) for patients with PSMA-expressing mCRPC who experienced disease progression despite prior treatment with a novel androgen axis drug inhibitor.
TLX591 is a first-in-class radio-antibody drug conjugate (rADC) for prostate-specific membrane antigen (PSMA)-expressing mCRPC.
TLX591 utilizes a PSMA-targeted monoclonal antibody (mAb) approach. mAbs are highly specific with low rates of off-target organ exposure, demonstrate prolonged retention in PSMA-positive tumors, and have a predictable safety profile. Two treatments are administered 14 days apart. This minimizes the occurrence of off-target side effects while delivering a meaningful therapeutic index. TLX591 has been evaluated in >240 patients across eight phase I and II studies and has demonstrated proven anti-tumor effects with overall survival benefits. In a phase I/II study of 49 men with mCRPC refractory to or refusing standard treatment options, TLX591 demonstrated any PSA decrease in 88% of patients with a median survival of 42.3 months.1 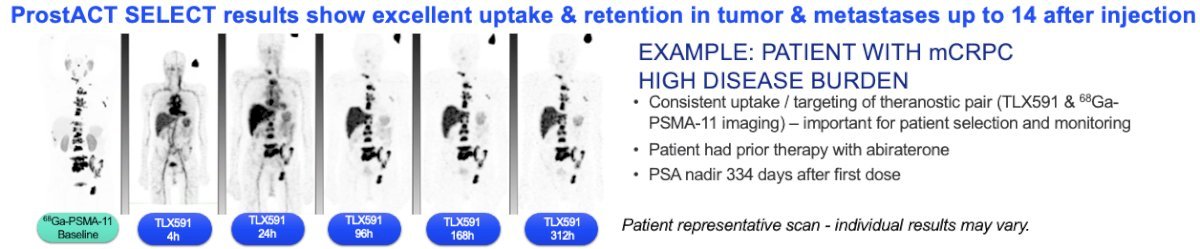
ProstACT GLOBAL is a prospective, open-label phase III trial of 430 patients with PSMA-expressing mCRPC who have experienced disease progression on prior androgen receptor pathway inhibitor (abiraterone acetate or enzalutamide only) received in either the metastatic castrate sensitive (de novo or recurrent) or first-line mCRPC treatment setting. Patients may have received docetaxel in the castrate-sensitive setting provided that the last dose of therapy was ≥6 months prior to screening.
The study design is summarized below. Part 1 will enroll 30 patients in the safety and dosimetry lead-in phase. The primary endpoints are biodistribution/pharmacokinetics, dosimetry, safety, and tolerability. In part 2 (randomized treatment expansion), 400 patients will be randomized 2:1 to either TLX591 + SOC versus SOC alone. The primary endpoint is radiographic progression-free survival. The key secondary endpoints include 5-year overall survival, tumor objective response rate, time to symptomatic skeletal event, health-related quality of life, and treatment-related adverse events count. 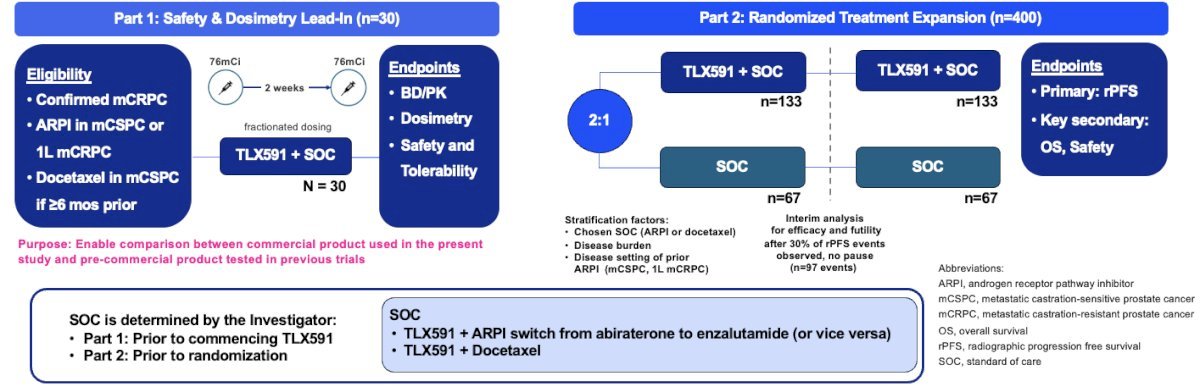
SOC is determined by the investigator prior to commencing TLX591 in Part 1 and prior to randomization in Part 2. 
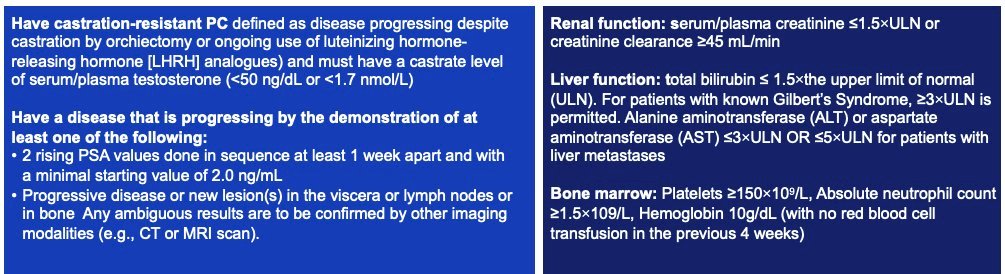
The key inclusion criteria are as follows:
- Adult patients with histologically/pathologically confirmed mCRPC (≥1 metastatic lesion on baseline CT, MRI, or bone scintigraphy) with PSMA-positive disease on a 68Ga-PSMA-11 PET/CT scan
- ECOG Performance Status 0–2 with an estimated life expectancy ≥6 months
- Previously treated with first androgen receptor pathway inhibitor (abiraterone or enzalutamide) in the mCSPC (de novo or recurrent) or 1st line mCRPC setting for ≥12 weeks.
- Must have recovered to Grade ≤2 from all clinically significant toxicities related to prior therapies
The key exclusion criteria are as follows:
- Pathological findings consistent with small cell or any histology other than adenocarcinoma of the prostate. Minor (<20%) elements of neuroendocrine histology is acceptable.
- Diagnosed with other malignancies that are expected to alter life expectancy or interfere with disease assessment.
- At increased risk of hemorrhage or bleeding, or with a recent history (within last 6 months) of a thrombolytic event
- Has received prior treatment with mAB J591 or any other PSMA-targeted therapy
- Has known brain, liver, lytic bone, or lymph node metastases ≥1cm in diameter
- Has a history of seizure and/or stroke within the past 6 months
- Has clinical or radiologic findings indicative of impending spinal cord compression
- Has evidence of a serious active or sub-clinical infection or angina pectoris, significantly prolonged QT interval, or other serious illness(es) involving the cardiac, respiratory, central nervous system, renal, hepatic, or hematological organ systems, that might impair the ability to complete this study or could interfere with determination of causality of any adverse effects experienced in this study, or which require treatment that could interact with study treatment, particularly with enzalutamide
- Has received treatment with any PARP inhibitors or with any platinum-based anti-neoplastic drugs
This study is currently ongoing.
Presented by: Scott T. Tagawa, MD, MS, FACP, Professor of Medicine and Urology, Weill Cornell Medicine, New York, NY
Written by: Rashid K. Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX between December 3rd and 6th, 2024
References:

