(UroToday.com) The 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX between December 3rd – 6th, 2024 was host to a prostate cancer poster session. Dr. Neeraj Agarwal presented the MEVPRO-1, a randomized phase III trial evaluating mevrometostat (PF-06821497) in combination with enzalutamide in patients with metastatic castration-resistant prostate cancer (mCRPC) previously treated with abiraterone acetate.
Resistance to androgen receptor (AR) pathway inhibitors (ARPI; e.g., abiraterone, enzalutamide) in mCRPC may be driven by preservation of AR signaling through various mechanisms.1–3 Enhancer of zeste homolog 2 (EZH2) is implicated in the pathogenesis of prostate cancer and ARPI resistance through several pathways including its function to silence tumor suppressor genes, activation of AR transcription factors, and promotion of neuroendocrine transdifferentiation.4-6 Thus, combining ARPIs with therapies that modulate alternative signaling pathways, including epigenetic modifiers such as EZH2, could be a promising treatment approach to overcome resistance.
Mevrometostat (PF-06821497) is a potent and selective small molecule EZH2 inhibitor.7 Enzalutamide is an ARPI approved for the treatment of patients with mCRPC, nonmetastatic CRPC, metastatic castration-sensitive prostate cancer (CSPC), and nonmetastatic CSPC with biochemical recurrence at high risk for metastasis.8 As there are currently no established guidelines for the sequencing of treatments post first-line ARPI (e.g., abiraterone) in mCRPC, clinical decisions are primarily based on factors such as prior treatments received, and the patient’s clinical and disease characteristics.9 In real-world settings, many patients whose disease has progressed on abiraterone treatment—particularly patients who are unsuitable for chemotherapy—receive a second ARPI. The clinical benefit of this sequence may be limited by cross-resistance.11 However, studies, including a systematic review, have demonstrated that the switch from abiraterone to enzalutamide is associated with greater prostate-specific antigen responses and a trend to improvement in overall survival,12,13 thus, allowing physicians the flexibility of choosing a treatment option that is appropriate for the individual patient.
Results from a dose-expansion study (NCT03460977) showed promising activity and a manageable adverse-event profile for mevrometostat combined with enzalutamide in patients with CRPC who had evidence of cancer progression and had received prior abiraterone and/or enzalutamide.14 In his presentation, Dr. Agarwal described the study design of a phase 3 trial that aims to evaluate whether the addition of mevrometostat to enzalutamide can reverse anti-androgen resistance, thereby increasing the duration of clinical benefit in patients with mCRPC previously treated with abiraterone
MEVPRO-1 (NCT06551324) is a global, randomized, open-label, phase III trial in mCRPC patients who were previously treated with abiraterone for ≥12 weeks. The key inclusion and exclusion criteria are detailed in the table below: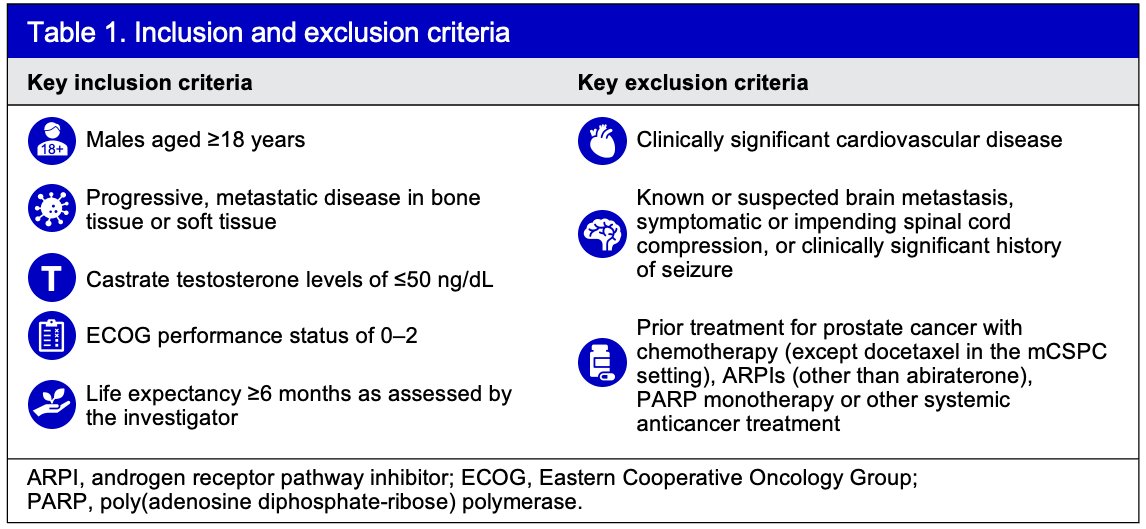
Approximately 600 patients will be randomized 1:1 to receive mevrometostat (875 mg twice daily with food) in combination with enzalutamide (160 mg once daily), or physician’s choice of enzalutamide (160 mg once daily) or docetaxel (75 mg/m2 intravenously every 21 days). The physician’s choice of treatment in the control group will be pre-specified prior to randomization. The sample size estimation is based on the number of events needed to observe protocol-defined statistical differences between the treatment groups.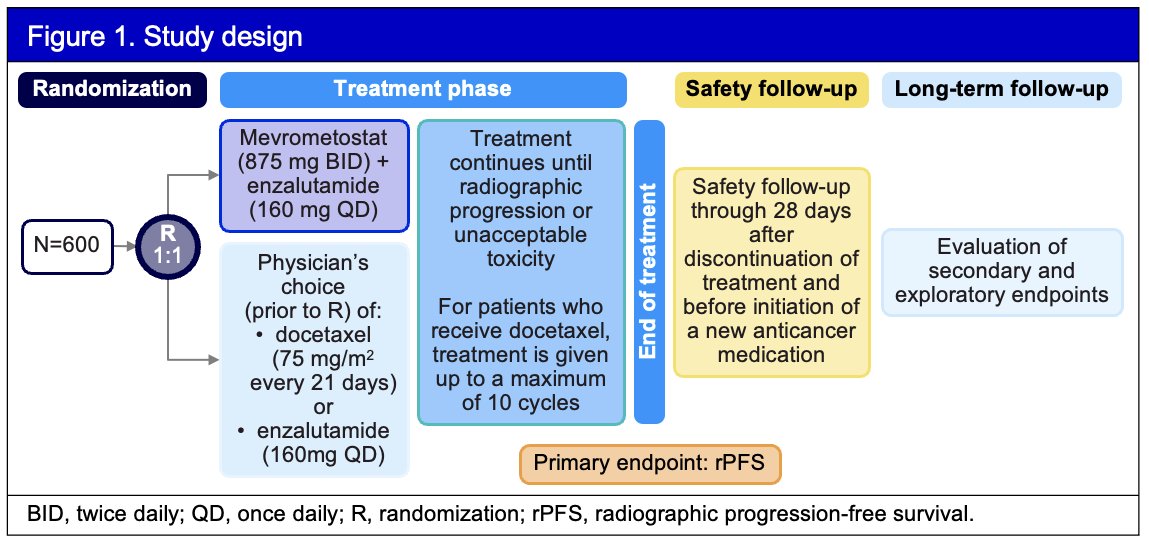
Randomization will be stratified by:
- Previous docetaxel in the mCSPC setting
- Physician’s choice of comparator (enzalutamide or docetaxel) prior to randomization
- Presence of hepatic metastases
The primary efficacy endpoint is radiographic progression-free survival (rPFS), per Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 (soft tissue) and Prostate Cancer Working Group 3 (PCWG3, bone) assessed by blinded central radiology review. The key secondary endpoint is overall survival; other secondary endpoints include measures of antitumor activity by overall response rate and duration of response, patient-reported outcomes, pharmacokinetics, and circulating tumor DNA burden. Safety will be assessed via adverse event monitoring, physical examinations, vital signs, and clinical laboratory tests.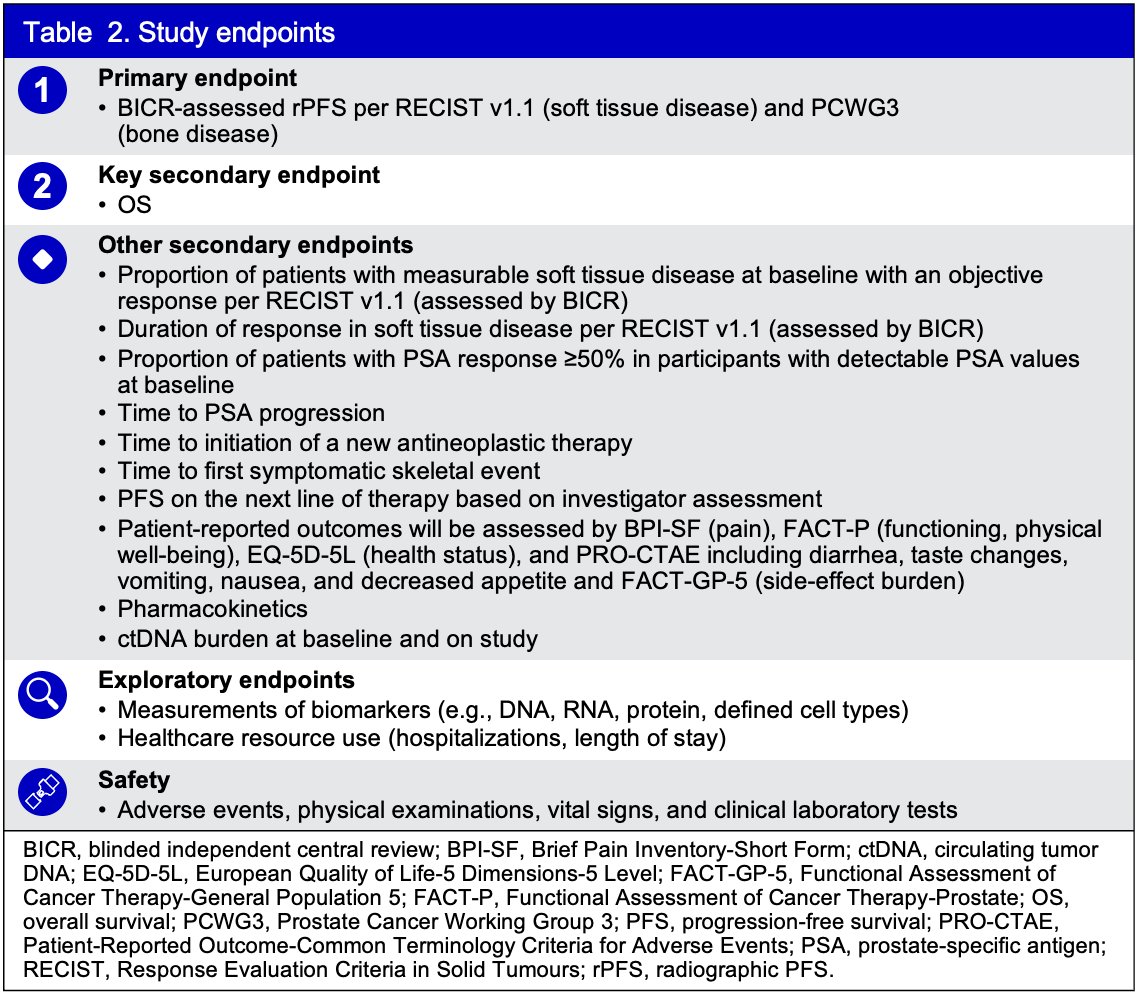
The statistical analysis approach is summarized below:

The 1st patient was enrolled into the study on October 21, 2024. In total, five countries are enrolling patients in MEVPRO-1. The study is estimated to be completed in October 2028.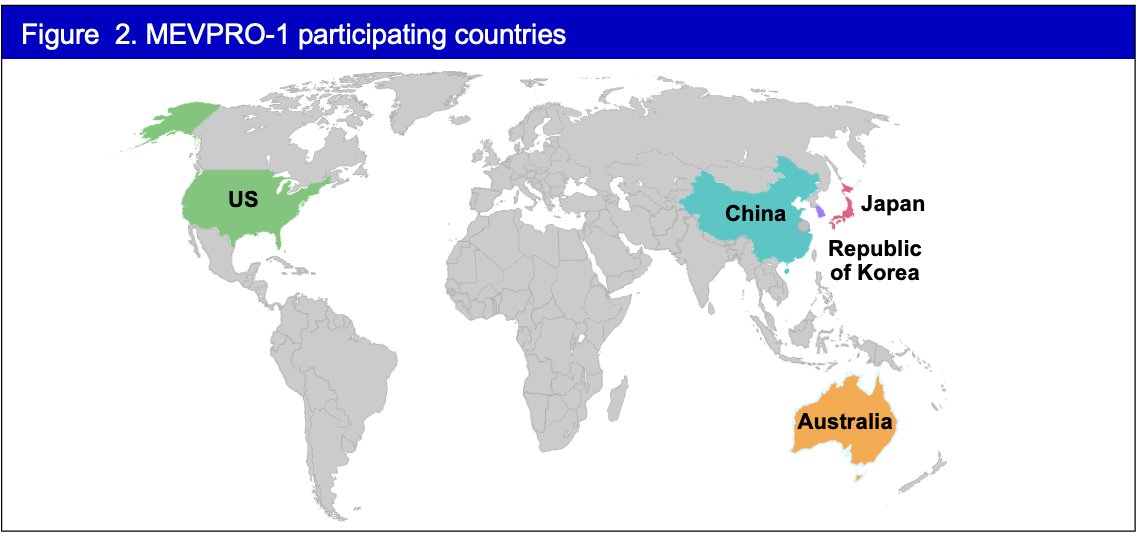
Presented by: Neeraj Agarwal, MD, FASCO, Professor, Department of Medicine, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT
Written by: Rashid K. Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX between December 3rd and 6th, 2024
References:
- Armenia J, Wankowicz SAM, Liu D, et al. The long tail of oncogenic drivers in prostate cancer. Nat Genet. 2018;50(5):645-651.
- Quigley DA, Dang HX, Zhao SG, et al. Genomic hallmarks and structural variation in metastatic prostate cancer. Cell. 2018;174(3):758-769.e9.
- Wedge DC, Gundem G, Mitchell T, et al. Sequencing of prostate cancers identifies new cancer genes, routes of progression, and drug targets. Nat Genet. 2018;50(5):682-692.
- Beltran H, Prandi D, Mosquera JM, et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Clin Cancer Res. 2019;25(23):6916-6924.
- Ku SY, Rosario S, Wang Y, et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science. 2017;355(6320):78-83.
- Berger A, Brady NJ, Bareja R, et al. N-Myc-mediated reprogramming drives lineage plasticity in advanced prostate cancer. J Clin Invest. 2019;129(9):3924-3940.
- Kung P-P, Snyder LB, Stabile MR, et al. Potent and selective phosphodiesterase 10A inhibitors for the treatment of schizophrenia. J Med Chem. 2018;61(3):650-665.
- Astellas Pharma US Inc. Full prescribing information: XTANDI® (enzalutamide) for oral use. https://www.astellas.us/docs/us/12A005-ENZ-WPI.pdf. Published 2023. Accessed November 6, 2024.
- Gillessen S, Attard G, Beer TM, et al. Management of patients with advanced prostate cancer: The Report of the Advanced Prostate Cancer Consensus Conference APCCC 2017. Eur Urol. 2018;73(2):178-211.
- de Bono JS, Fizazi K, Saad F, et al. Central results from a randomized, phase III trial of abiraterone acetate plus prednisone in patients with metastatic castration-resistant prostate cancer after progression on docetaxel: The COU-AA-301 trial. Eur Urol. 2018;74(1):37-45.
- George DJ, Srinivas S, Armstrong AJ, et al. Personalized oncology through integrating clinical and molecular information in advanced prostate cancer: The PROMOTE Study. Prostate Cancer Prostatic Dis. 2024;27(4):756-764.
- Chung DY, Kim S, Suh YE, et al. Comparative analyses of tumor immunity and immune cell characteristics among molecular subtypes of bladder cancer. Cancers (Basel). 2019;12(1):8.
- Khalaf DJ, Annala M, Taavitsainen S, et al. Phase 2 randomized clinical trial of bipolar androgen therapy for men with metastatic castration-resistant prostate cancer. Lancet Oncol. 2019;20(12):1730-1739.
- Schweizer MT, Gulati R, Montgomery B, et al. Early effects of androgen deprivation therapy and survival in men with localized prostate cancer: Results from the Scandinavian Prostate Cancer Group-4 trial. J Clin Oncol. 2024;42(16_suppl):5061.


