(UroToday.com) The 2024 SUO annual meeting included a prostate cancer session, featuring a presentation by Dr. Daniel George discussing real-world treatment and clinical outcomes in patients with mCRPC treated with olaparib in the United States. Olaparib is one of the first targeted therapies for patients with mCRPC. Data from the PROfound clinical trial1 showed that patients with homologous recombination repair (HRR) gene mutations who received olaparib monotherapy had a longer treatment duration (median 7.6 versus 3.9 months), improved progression free survival (median 5.8 versus 3.5 months), and improved overall survival (median 17.3 versus 14.0 months) compared to patients who received physician’s choice of either enzalutamide or abiraterone. This study describes the timing and type of biomarker testing relative to olaparib monotherapy use and the real-world time on treatment, real-world progression free survival, and real-world overall survival in patients with HRR mutation mCRPC treated with olaparib monotherapy.
This retrospective study used data abstracted from electronic medical records in the ConcertAI Oncology Dataset, which consists of de-identified EMR data drawn from geographically diverse practice locations within the US and are primarily community oncology practices (80%-90%) from both rural and urban centers. Patients with confirmed mCRPC, diagnosed between 2012 and 2023, age ≥21 years, treated with olaparib monotherapy (post-May 19, 2020) after exposure to abiraterone or enzalutamide, and with positive HRR mutation status were included. HRR genes of interest were ATM, BARD1, BRCA1, BRCA2, BRIP1, CDK12, CHEK1, CHEK2, FANCL, PALB2, RAD51B, RAD51C, RAD51D, RAD54L. The timing of biomarker testing was evaluated from date of first documented biomarker test to start date of earliest olaparib monotherapy (index date). Somatic and germline testing rates were reported. Kaplan-Meier analysis was used to estimate median real-world time on treatment, real-world progression free survival, and real-world overall survival from the index date. Patients were stratified by line of earliest olaparib monotherapy. Lines of therapy were evaluated from initial prostate cancer diagnosis.
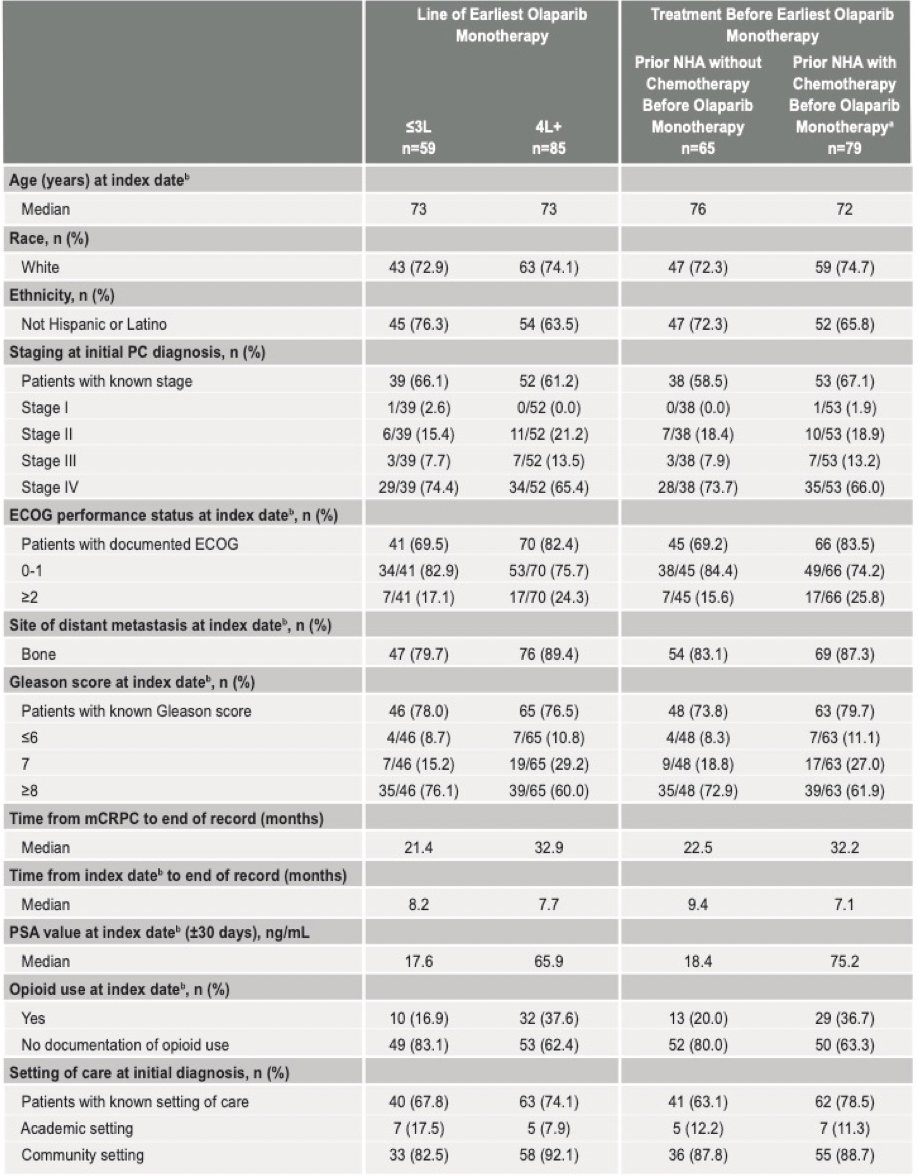
A total of 144 patients with HRR mutation mCRPC treated with olaparib monotherapy were identified, including 41.0% that initiated olaparib monotherapy in the first 3 lines, and 59.0% that initiated olaparib monotherapy in the fourth line and beyond. The median age was 73 years, 73.6% were white, 78.4% had ECOG performance status 0-1 at index, 66.7% had Gleason score ≥8 at index, 56.3% had a BRCA mutation including co-occurring HRR mutation, and the median time from index assessment was 8.1 months. The median time from index date to the end of record was 8.2 months for those receiving treatment in the first 3 lines, and 7.7 months for those receiving olaparib in the fourth line and beyond:
Overall, the median real world time on treatment for earliest olaparib monotherapy 4.6 months (95% CI 3.5 - 5.5), for patients with less than 3 lines of therapy was 5.3 months (95% CI 4.0 – 8.2), and for more than four lines of therapy was 3.8 months (95% CI 2.1 – 5.5):

Overall, the median real world progression free survival for earliest olaparib monotherapy 4.5 months (95% CI 3.6 - 5.6), for patients with less than 3 lines of therapy was 5.3 months (95% CI 3.6 – 6.8), and for more than four lines of therapy was 3.9 months (95% CI 3.2 – 5.5):

Overall, the median real world overall survival for earliest olaparib monotherapy 16.5 months (95% CI 12.4 - 21.2), for patients with less than 3 lines of therapy was 16.5 months (95% CI 11.7 – 24.1), and for more than four lines of therapy was 15.0 months (95% CI 11.0 – 21.0):

There were several limitations to this trial:
- The study outcomes were examined using Kaplan-Meier analysis and were descriptive in nature. Due to the small sample sizes, the analyses did not include adjusted models to control for confounders
- Patients within the ConcertAI Oncology Dataset may differ from the underlying mCRPC population in ways that may not be measurable. Findings from this study should be generalized only to the underlying population who meet the study eligibility criteria
- The index date for all patients was from the initiation of olaparib monotherapy for all clinical endpoints, including real world overall survival. Outcomes were measured from the time of randomization in the PROfound clinical trial
- Although this study includes some patients treated in academic settings, most were treated in community oncology practices, and were all treated in the US. Treatment patterns may differ in academic centers compared with community settings or in practices outside of the US
- The results of this study should be interpreted in consideration of its retrospective design and the known limitations of chart review. The analyses were, therefore, limited to some extent by the availability of data in the database. Due to provider documentation practices, certain variables may have slightly varying definitions within different patient records in the ConcertAI Oncology Dataset
Dr. George concluded his presentation by discussing real-world treatment and clinical outcomes in patients with mCRPC treated with olaparib in the United States with the following take home messages:
- This analysis reports the real world biomarker testing and treatment-related outcomes associated with olaparib monotherapy in patients with HRR mutation positive mCRPC
- Many patients received olaparib monotherapy in later lines of therapy several months after initial HRR mutation testing, and they may not fully realize the clinical benefit of olaparib
- The real world progression free survival and overall survival results showed similar results to the PROfound clinical trial despite later line use in the real world setting
- The results suggest that patients may benefit from improved outcomes with earlier biomarker testing and would allow for earlier treatment with targeted therapy in the disease course when appropriate
Presented by: Daniel J. George, MD, Duke University, Durham, NC
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Society of Urologic Oncology (SUO) Annual Meeting, Dallas, TX, Tues, Dec 3 – Fri, Dec 6, 2024.
References:


