(UroToday.com) The 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX was host to a prostate cancer poster session. Dr. Mohamed Ahmed presented the results of a study assessing response to 177Lu-PSMA-617 by disease site in metastatic castrate-resistant prostate cancer (mCRPC) patients.
In March 2022, the United States Food and Drug Administration (FDA) approved Pluvicto (177Lu-PSMA) for the treatment of adult patients with prostate-specific membrane antigen (PSMA)-positive mCRPC who have been treated with an androgen receptor pathway inhibitor and taxane-based chemotherapy,1 based on the results of the VISION and TheraP trials.2,3
Since then, numerous studies have evaluated the response to 177Lu-PSMA; however, few have described the treatment response based on the site of disease progression. In this study, Dr. Ahmed and colleagues sought to assess the response to 177Lu-PSMA based on the site of metastatic disease.
This was a retrospective analysis of 273 patients who were treated with 177Lu-PSMA at The Mayo Clinic from April 2022 to September 2023. Of these 273 patients, 76 (28%) were noted to have presented with either bone-only or lymph node-only disease. Clinicopathologic variables were extracted by trained research personnel. Follow-up imaging and clinical documentation were used to record the treatment outcomes. Statistical comparisons of categorical and continuous variables were performed using the Chi-square and Kruskal-Wallis tests, respectively.
These 76 patients were categorized into one of two groups according to the site of disease progression: pure bone disease (52 patients; 68%) and pure lymph node disease (24 patients; 32%) without any other sites of disease progression, and both had the same volume of disease. Patients in the bone group had a higher median Gleason Score at diagnosis (8 versus 7, p=0.02) and PSA levels at 177Lu-PSMA initiation (20.1 versus 2.9 ng/ml, p=0.016).
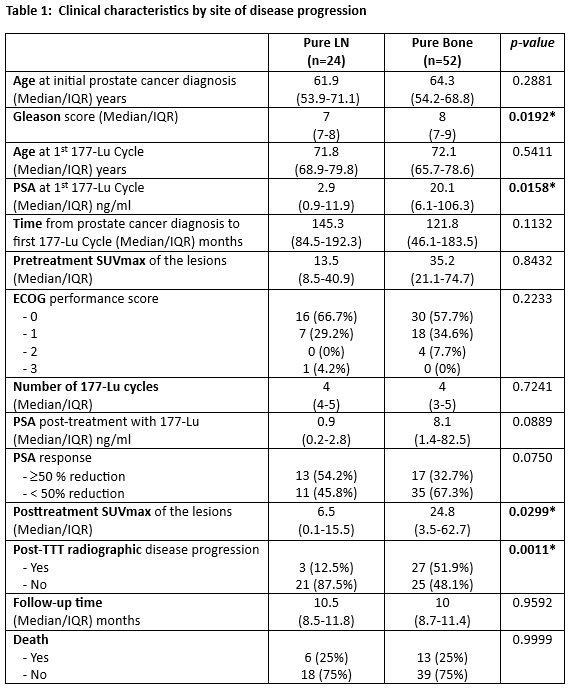
At a median follow-up of 10.2 (IQR: 8.6–11.5) months from the first 177Lu-PSMA cycle, differences were noted in the PSA response kinetics. Patients in the lymph node group were more likely to have a PSA50 response (54% versus 33%, p=0.075), with lower median PSA levels following treatment (0.9 versus 8.1 ng/ml, p=0.09). Additionally, the post-treatment SUVmax of the lymph node lesions remained lower post-treatment (6.5 versus 24.8, p=0.03), consistent with what was observed pre-treatment (13.5 versus 35.2). Patients in the lymph node group were less likely to experience radiographic disease progression (12.5% versus 52%, p=0.96).
Based on these results, Dr. Ahmed concluded that patients who were treated with 177Lu-PSMA and had lymph node-only disease experience a more durable response compared to those with bone-only disease.
Presented by: Mohamed E. Ahmed, MD, Urology Resident Physician, Department of Urology, Mayo Clinic, Rochester, MN
Written by: Rashid K. Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, between the 3rd and 6th of December, 2024.
References:
- FDA approves Pluvicto for metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pluvicto-metastatic-castration-resistant-prostate-cancer. Accessed on December 4, 2024.
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021; 385(12):1091-1103.
- Hofman MS, Emmett L, Sandhu S, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomized, open-label, phase 2 trial. Lancet. 2021; 397(10276):797-804.


