(UroToday.com) The 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, between December 3 and December 6, 2024, was host to the Abstract/Posters Session. Dr. Evan Yu and John Murray presented a phase 3 study of CYP11A1 inhibitor Opevesostat versus next-generation hormonal agent (NHA) switch in metastatic castration-resistant prostate cancer after NHA and taxane-based chemotherapy.
The androgen receptor (AR) plays a crucial role in prostate cancer progression, including in metastatic castration-resistant prostate cancer (mCRPC). Despite androgen deprivation therapies (ADT), AR signaling remains activated in many patients with mCRPC, often due to somatic mutations in the AR ligand-binding domain (AR-LBD). These mutations, which occur in 20-25% of patients treated with novel hormonal agents, contribute to resistance to such therapies.(1)
Opevesostat (MK-5684, ODM-208) is an oral, nonsteroidal drug that inhibits cytochrome P450 11A1 (CYP11A1), the enzyme responsible for the first and rate-limiting step of steroid biosynthesis. By inhibiting CYP11A1, opevesostat effectively reduces the production of steroid hormones and their precursors, which could otherwise activate the AR signaling pathway and promote cancer progression. This mechanism targets the broader steroidogenic pathway, aiming to suppress AR-driven resistance in mCRPC.
In patients with heavily pretreated mCRPC, and particularly in those with AR-LBD mutations, Opevesostat showed antitumor activity in the phase 1/2 CYPIDES trial. (2)
The aim of this presentation is to report the design of the randomized, open-label, phase 3 MK-5684-003 study (NCT06136624) evaluating the efficacy and safety of opevesostat versus NHA switch in patients with mCRPC who received at least two lines of therapy: NHA and taxane-based chemotherapy.
The randomization for this study will be stratified by:
(1) presence or absence of measurable disease
(2) detection of AR-LBD mutations in circulating tumor DNA
(3) prior receipt of cabazitaxel
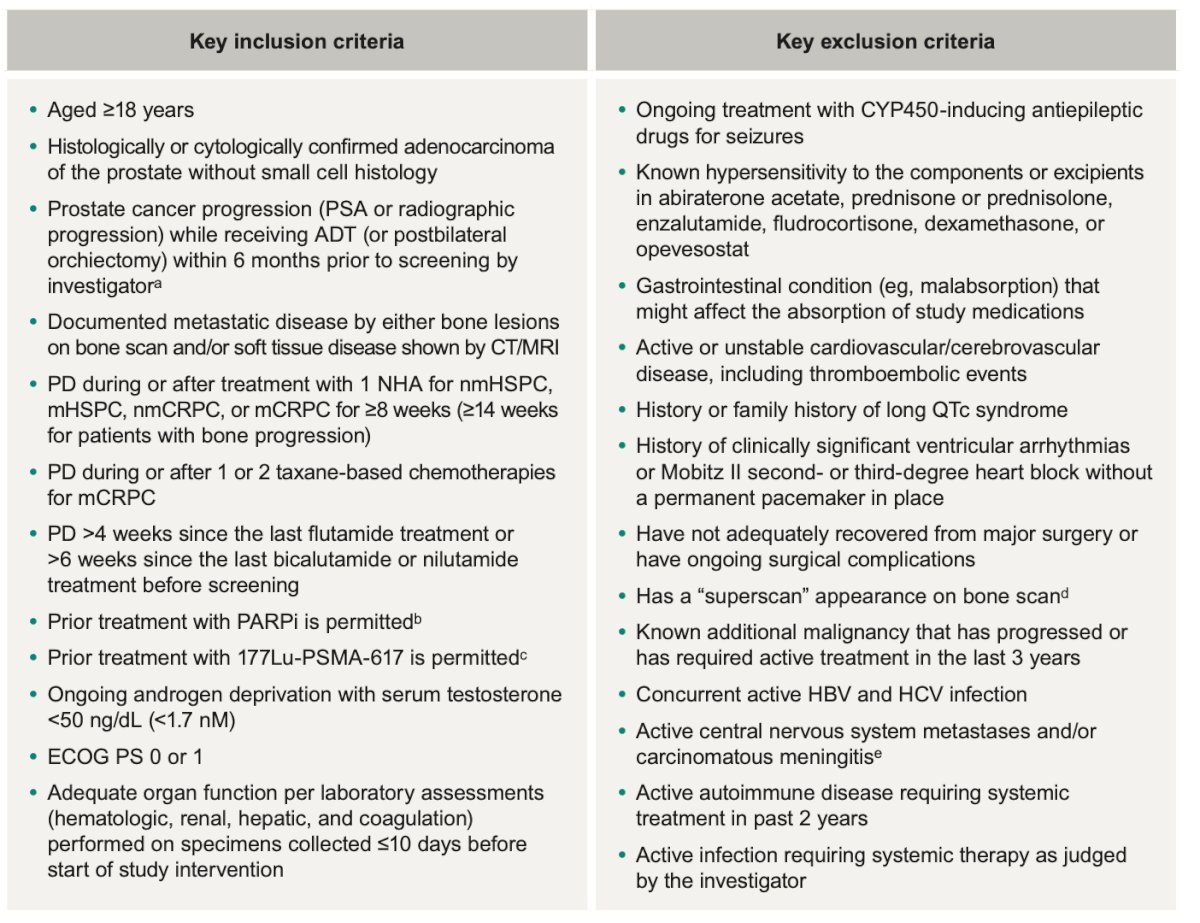
The inclusion and exclusion criteria for the study are summarized in the table below:
Primary
- Radiographic progression-free survival (rPFS) per Prostate Cancer Clinical Trials Working Group 3 (PCWG3)–modified RECIST v1.1 by blinded independent central review (BICR)
- Overall survival (OS)
Secondary
- Time from randomization to initiation of the first subsequent anticancer therapy or death (TFST)
- Objective response rate (ORR) and duration of response (DOR) per PCWG3-modified RECIST v1.1 by BICR in patients with measurable disease
- Time to pain progression (TTPP; time from randomization to pain progression as determined by item 3 of the Brief Pain Inventory–Short Form [BPI-SF] and by the Analgesic Quantification Algorithm score)
- Time to prostate-specific antigen (PSA) progression (time from randomization to the date of PSA progression)
- Time to first symptomatic skeletal-related event (SSE; time from randomization to the first occurrence of an SSE)
- Safety and tolerability
The study design is shown below. Briefly, patients will be randomly assigned 1:1 to:
- Opevesostat 5 mg PO BID (+ dexamethasone 1.5 mg and fludrocortisone 0.1 mg QD) or enzalutamide 160 mg PO QD (if prior abiraterone) or
- Abiraterone acetate 1000 mg PO QD (if prior enzalutamide/darolutamide/apalutamide).
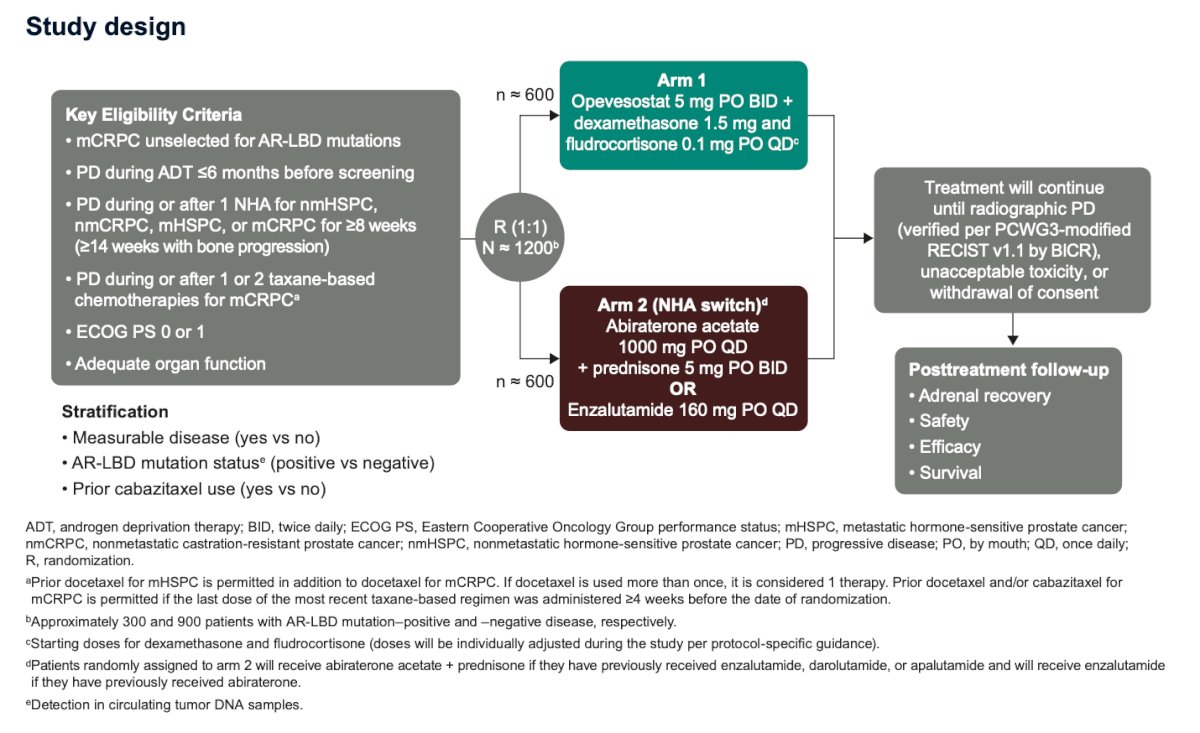
The follow-up assessment for patients treated with opevesostat (ODM-208) will focus on five key categories: tumor response, PSA (prostate-specific antigen) levels, adverse events, adrenal recovery, and patient-reported outcomes (PROs). These parameters will provide a comprehensive evaluation of the treatment's efficacy and safety. The specific follow-up schedules for monitoring these outcomes are detailed in the table below:
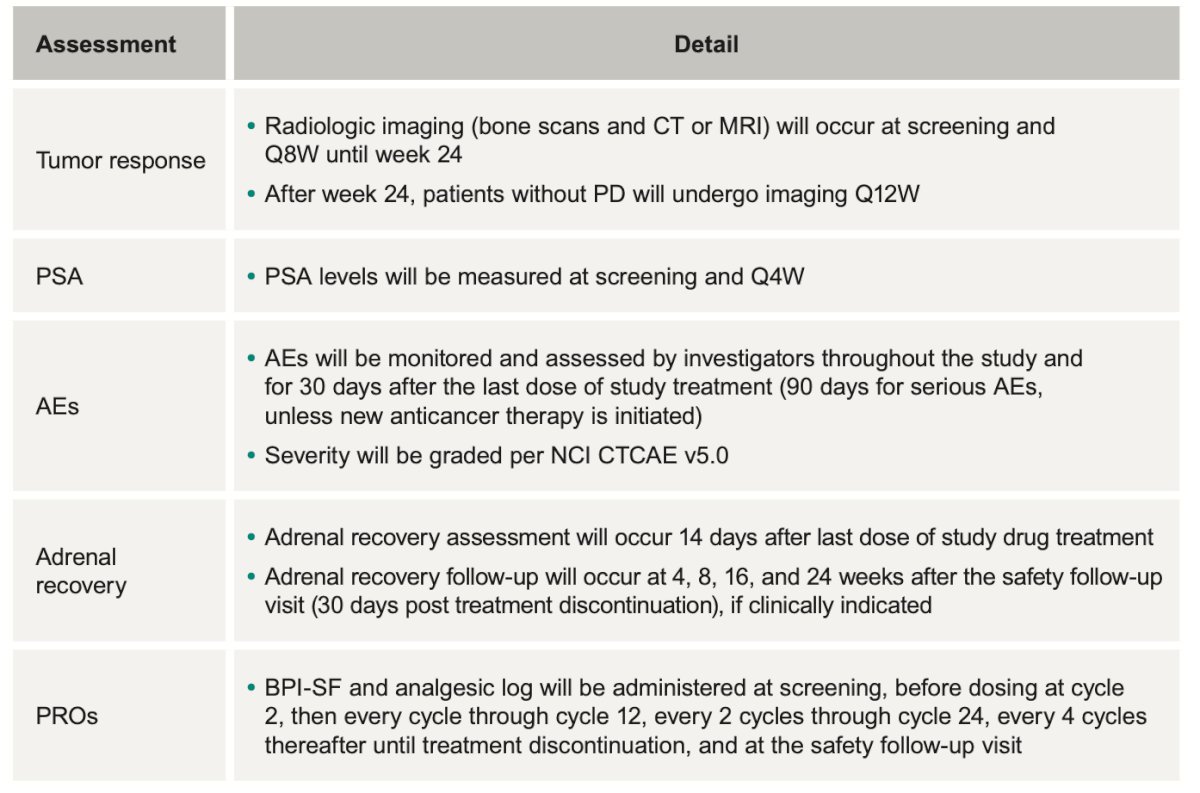
There will be three different sets of analysis. The efficacy analysis will be conducted in the intention to treat population. The safety analysis in all randomly assigned patients who received ≥1 dose of study treatment and the PROs analysis in all randomly assigned patients who received ≥1 dose of study treatment and have ≥1 PRO assessment available.
The MK-5684-003 study (NCT06136624) is currently ongoing, and the sites of enrollment are illustrated in the figure below:
Presented by: Evan Yu, MD, Medical Oncologist, Section Head, Medical Oncology, Clinical Research Division, Fred Hutch Cancer Centre, Seattle, WA.
Written by: Julian Chavarriaga, MD –Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, between the 3rd and 6th of December, 2024.
References:- Fizazi K, Bernard-Tessier A, Roubaud G, Utriainen T, Barthélémy P, Fléchon A, van der Voet J, Gravis G, Ratta R, Jones R, Parikh O, Tanner M, Antonarakis ES, Baldini C, Peters N, Garratt C, Ikonen T, Pohjanjousi P, Joensuu H, Cook N. Targeted Inhibition of CYP11A1 in Castration-Resistant Prostate Cancer. NEJM Evid. 2024 Jan;3(1):EVIDoa2300171. doi: 10.1056/EVIDoa2300171. Epub 2023 Dec 26. Erratum in: NEJM Evid. 2024 Feb;3(2):EVIDx2300368. doi: 10.1056/EVIDx2300368. PMID: 38320513; PMCID: PMC10852404.
- Karimaa M, Riikonen R, Kettunen H, Taavitsainen P, Ramela M, Chrusciel M, Karlsson S, Rummakko P, Simola O, Wohlfahrt G, Hakulinen P, Vuorela A, Joensuu H, Utriainen T, Fizazi K, Oksala R. First-in-Class Small Molecule to Inhibit CYP11A1 and Steroid Hormone Biosynthesis. Mol Cancer Ther. 2022 Dec 2;21(12):1765-1776. doi: 10.1158/1535-7163.MCT-22-0115. PMID: 36129801.


