(UroToday.com) The 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX between December 3rd – 6th, 2024 was host to a prostate cancer session. Dr. Mohit Khera discussed the use of testosterone supplementation after treatment of localized prostate cancer.
Dr. Khera began by noting that over the past 20 years, there seems to be a paradigm shift in our perception of testosterone therapy and prostate cancer, as outlined below:
Summarized in the table below are the aggregates of studies evaluating testosterone therapy in localized prostate cancer patients. Overall, there only 30 published studies to date, including a total of <1,000 patients.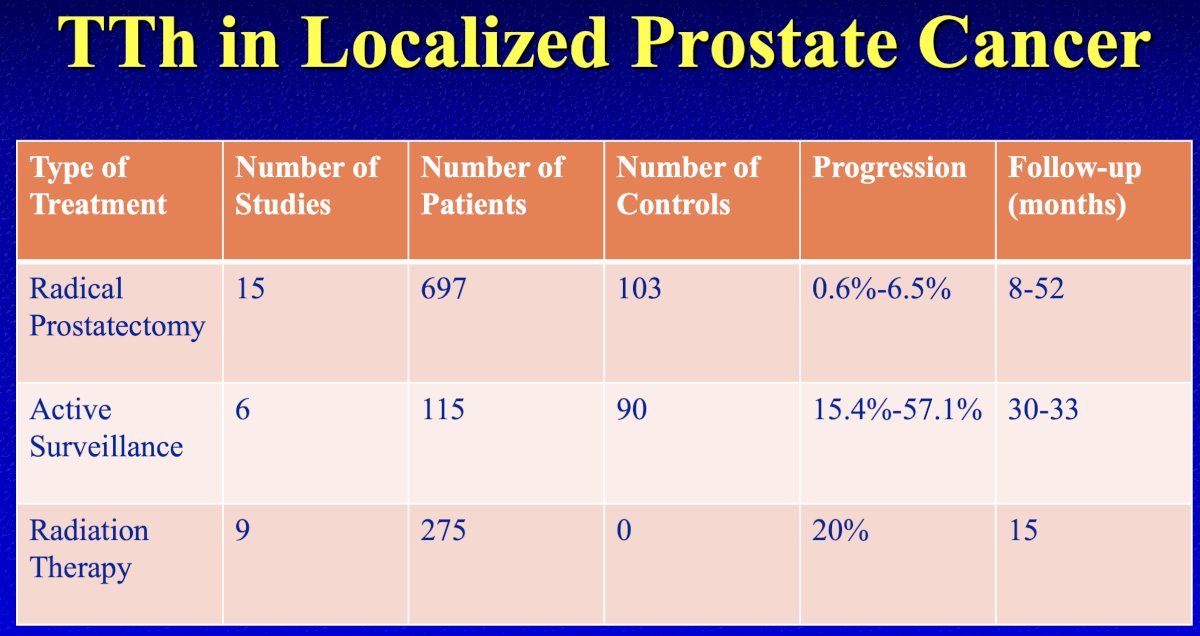
In the post-radical prostatectomy setting, there are 15 published studies to date, which included a total of 697 testosterone replacement therapy (TRT)-treated patients and 103 TRT-untreated controls. Overall, these studies suggest that TRT might be harmless in patients following a radical prostatectomy (progression rate: 0–6.5% during 8–52 months of follow-up). However, it is important to note that all included studies were case reports or case series, except for two double-armed retrospective studies, with poor quality scores (median MINOR score, 10 [range: 4–16]).1
In the post-radiation therapy setting, there are nine published studies, which include a total of 275 TRT-treated patients. All published studies to date in this setting are case series, and, as such, there are no TRT-untreated controls. The results of one study implied that TRT might have harmful effects on the prognosis of patients who had received prior radiation therapy, with a progression rate of 20% during the 15 months follow-up.1 However, Dr. Khera did note that he believes that these numbers may be artificially inflated.
Existing evidence for TRT in patients with active surveillance comprises six studies, which include a total of 115 TRT-treated patients and 90 TRT-untreated controls. Among the six included studies, only one was designed as a double-armed study, and all others were case reports or case series. The median quality score of the six studies was as low as 9 (range: 5–15). Of the six studies, the results of two implied that TRT might have harmful effects on the prognosis of patients with active surveillance (progression rate: 15.4%–57.1% during 30–33 months of follow-up).1
Next, Dr. Khera highlighted a study published by his group in 2015. This multi-institutional study included 98 hypogonadal men treated with TRT following either external beam radiotherapy or brachytherapy, and, to date, represents the largest series of TRT following radiation therapy for prostate cancer patients. The median follow-up in this 2015 study was 40.8 months. 77% of the cohort had low or intermediate risk prostate cancer. A clinically insignificant increase in mean PSA was noted from 0.08 ng/ml to 0.09 ng/ml; however, among high-risk patients, the mean PSA increased from 0.10 to 0.36 ng/dL. Six (6.1%) men met either the Phoenix or American Society for Radiation Oncology (ASTRO) criteria for biochemical recurrence during the study period, with two of those requiring subsequent initiation of ADT. Two of these patients were treated with brachytherapy and may have been experiencing a ‘PSA bounce’, rather than true biochemical recurrence. The authors noted that a 6% biochemical recurrence rate would indeed be lower than previously reported rates for radiation therapy, although the limited sample size, retrospective study design, and single-arm cohort makes drawing definitive conclusions challenging.2
The use of TRT for prostate cancer patients is supported by the ‘Prostate Saturation Model’. This model suggests that prostate growth is exquisitely sensitive to variations in androgen concentrations at very low concentrations but becomes insensitive to changes in androgen concentrations at higher levels. This pattern is consistent with the observation that androgens exert their prostatic effects primarily via binding to the androgen receptor, and that maximal androgen-androgen receptor binding is achieved at serum testosterone concentrations well below the physiologic range.3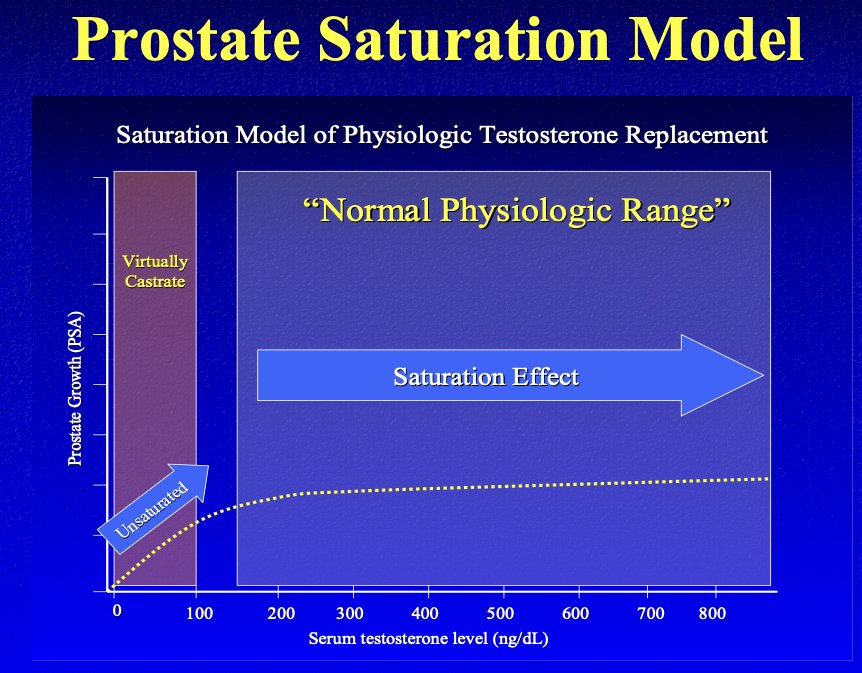
The validity of this model is supported by the results of an observational study published by Dr. Khera’s group in 2011. In this study, 451 hypogonadal men were started on TRT for 12 months and were divided into two groups based on the pre-TRT serum testosterone level:
- Group A: Testosterone <250 ng/dL
- Group B: Testosterone ≥250 ng/dL
After 12 months of TRT, a significant increase in serum PSA levels was observed only in Group A (baseline testosterone <250 ng/dL). Importantly, the highest PSA was observed 1 month following treatment initiation and was correlated with both total and free testosterone levels.4
In 2024, Flores et al. published the results of a series of radical prostatectomy patients with Grade Group 1–3 organ confined disease who received TRT (n=198), along with 5,001 controls who did not receive TRT. The study investigators evaluated time to biochemical recurrence following TRT initiation, with biochemical recurrence defined as a serum PSA level ≥0.1 ng/ml, confirmed on a repeat measurement.
The study investigators demonstrated a non-significantly decreased rate of biochemical recurrence with the use of TRT following radical prostatectomy (HR: 0.84; 95% CI: 0.48–1.46; p=0.5). The overall rates of biochemical recurrence were low, with 5-year rates of <2% in both groups.5
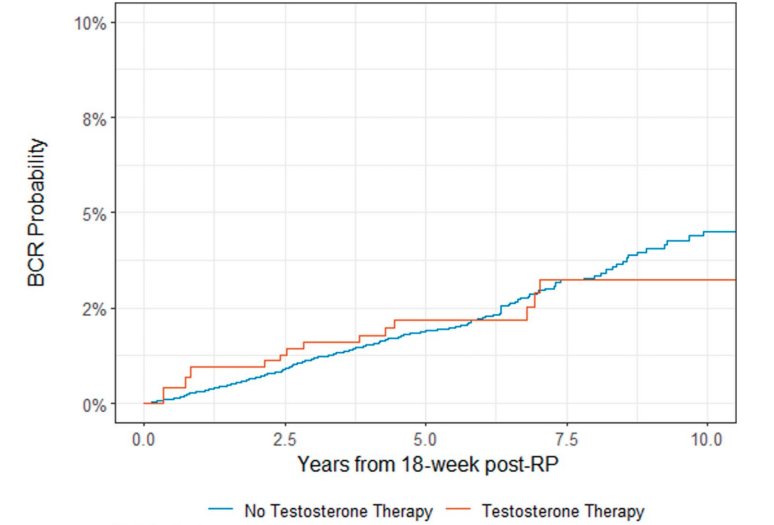
What about biochemical recurrence following TRT initiation in high-risk prostatectomy patients? Data from the Memorial Sloan Kettering Cancer Center demonstrates that the overall proportion of such patients who develop biochemical recurrence is ~73%, with a mean time to recurrence of 19 months.
But can TRT somehow be associated with more favorable/less aggressive prostate cancer variants? In 2017, Loeb et al. published the results of a nested case-control study from the National Prostate Cancer Register of Sweden, which included all 38,570 prostate cancer cases diagnosed between 2009 and 2012, along with 192,838 age-matched men free of prostate cancer. Overall, 284 patients with prostate cancer (1%) and 1,378 control cases (1%) filled prescriptions for TRT. There was no association found between TRT and the overall prostate cancer risk (OR: 1.03, 95% CI: 0.9–1.17). Interestingly, patients who received TRT had lower odds of aggressive prostate cancer (OR: 0.50; 95% CI: 0.37–0.67). The authors concluded that ‘the decrease in risk of aggressive prostate cancer with TRT is a novel finding that warrants further investigation”.6 Dr. Khera noted that this is not a placebo-controlled trial, and there are important limitations and detection biases inherent to such studies.
Additional benefits of TRT may include slowing recurrence rates in low-risk prostate cancer patients. In 2020, Ahlering et al. published the results of a series of 850 patients who underwent a radical prostatectomy, 152 of whom had low-risk disease without evidence of disease recurrence and received TRT. At a median follow-up of 3.5 years, disease recurrence was observed in 7.2% of TRT-treated patients, compared to 12.6% of those who did not receive TRT. Overall, patients receiving TRT were 54% less likely to experience disease recurrence, and among those who did recur, TRT was associated with a delayed time to recurrence of 1.5 years. Whether this represents a true biologic phenomenon versus a selection bias remains to be determined.7
There is currently an ongoing randomized, placebo-controlled trial (NCT00848497) that is evaluating TRT in hypogonadal men 3 months post-radical prostatectomy.8 Eligible patients must have undergone a bilateral nerve spare, and nadir PSA values are required to be <0.01ng/ml on 2 consecutive measurements (4 weeks apart). The exclusion criteria are as follows:
What about the impact of testosterone levels at diagnosis? In other words, is there a difference in underlying biology/tumor aggressiveness of tumors that develop in hypogonadal versus eugonadal patients? A retrospective study by Ferro et al. published in 2017 included 338 active surveillance-eligible patients who underwent a radical prostatectomy and met the following criteria: ≤cT2a, PSA <10 ng/ml, ≤2 cancer cores involved, Grade Group ≤1, and PSA density <0.2 ngml/cc. Low serum testosterone levels (i.e., <300 ng/dL) was demonstrated to be significantly associated with increased odds of:
- Upgrading to Grade Group ≥2 disease
- Upstaging to >pT2 disease
- Unfavorable disease (presence of >pT2 and/or predominant Gleason pattern 4 disease)
- Positive surgical margins
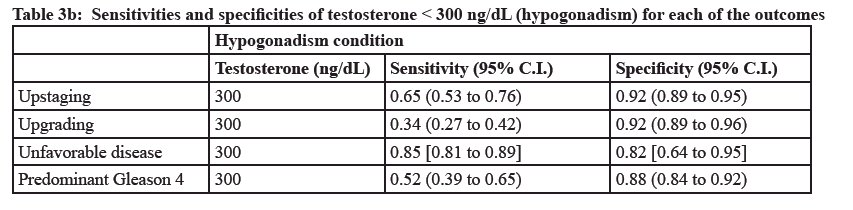
Based on these results, the study investigators concluded that: “Testosterone should be a selection criterion for inclusion of low-risk prostate cancer patients in active surveillance programs, and testosterone levels <300 ng/dL should be considered a discouraging factor when a close active surveillance program is considered as a treatment option.”9
What about the ‘prostate safety’ of TRT in hypogonadal men? In 2023, Bhasin et al. published the results of a randomized clinical trial that included 5,246 hypogonadal men (aged 45–80 years) with cardiovascular disease (CVD) or increased CVD risk, with the objective of comparing TRT to placebo. They demonstrated no differences in the incidences of/requirements for:10
- High grade prostate cancer (TRT group: 0.19%, placebo: 0.12%)
- Any prostate cancer (12 vs 11)
- Acute urinary retention (20 vs 16)
- Invasive surgical procedures (23 vs 12)
- Prostate biopsy (16 vs 14)
- New pharmacologic treatment (101 vs 87)
Dr. Khera noted that the role of TRT in prostate cancer continues to grow, even in advanced prostate cancer, where bipolar androgen therapy (BAT) has been evaluated in numerous clinical trials of castrate-resistant prostate cancer (CRPC). Prostate cancer cells adapt to chronic low testosterone conditions by upregulating androgen receptor activity leading to a 30 to 90-fold increase in androgen receptor levels. While this marked upregulation of the androgen receptor can cause castration resistance, it also creates a therapeutic vulnerability to treatment with supraphysiologic androgen, which can lead to growth arrest or cell death.11-19
The term ‘bipolar’ in BAT refers to the rapid cycling between two polar extremes: from supraphysiologic back to near-castrate serum testosterone levels. It can be achieved by administering testosterone cypionate at the US Food and Drug Administration (FDA)–approved dose of 400 mg intramuscularly once every 28 days, while maintaining continuous testosterone suppression via surgical castration or luteinizing hormone–releasing hormone (LHRH) agonists or antagonists.
Published in The Journal of Clinical Oncology in 2021, TRANSFORMER is a multicenter, open-label, phase II trial that randomized 195 men with mCRPC who had disease progression following treatment with abiraterone to either BAT (n=94) or enzalutamide 160 mg once daily (n=101). At a median follow-up time of 32 months, there was no difference in the primary study outcome, progression-free survival (BAT: 5.6 months versus enzalutamide: 5.6 months; HR: 1.13, p=0.45). There was similarly no difference in overall survival (BAT: 33 months versus enzalutamide: 29 months; HR: 0.95, p=0.80). A PSA50 decline was observed in 28% and 25% of patients in the BAT and enzalutamide arms, respectively.20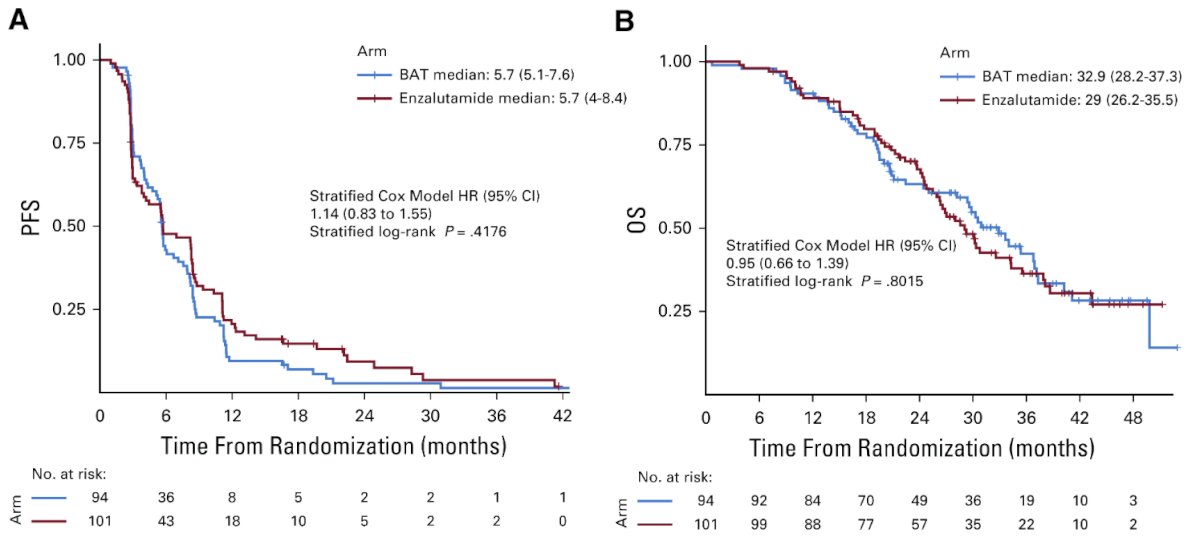
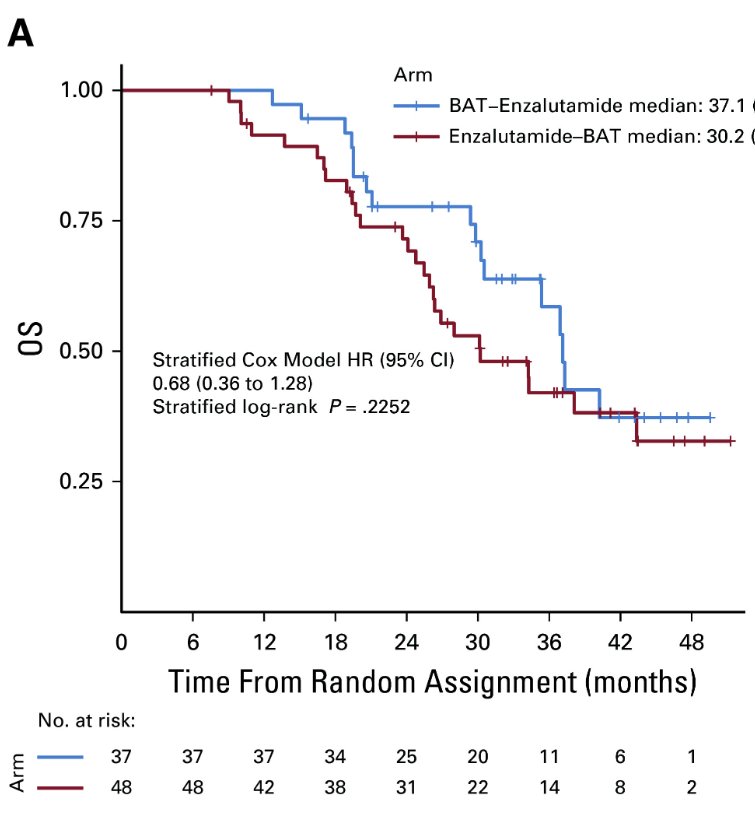
Patients who remained asymptomatic and continued to meet eligibility requirements were provided the opportunity to cross over, after a 28-day washout period, to the alternate treatment at time of progression. Most patients crossed over due to radiographic progression (90–95%). Crossover to enzalutamide following BAT was associated with greater benefits than crossover to BAT following enzalutamide. PSA50 responses occurred in 77.8% of patients crossing over to enzalutamide, compared to 23.4% in those crossing over to BAT. The progression-free survival from randomization through crossover (PFS2) was 28.2 months for BAT→enzalutamide versus 19.6 months for enzalutamide→BAT (HR: 0.44; p=0.02). The overall survival was 37.1 months for enzalutamide→BAT versus 30.2 months for enzalutamide→BAT (HR: 0.68, p=0.23).20
Dr. Khera concluded his presentation by making a case for early androgen use in post-prostatectomy hypogonadal men. He emphasized the importance of testosterone for enhancing the recovery of the cavernosal nerves, trabecular smooth muscle, and endothelium, all of which are crucial components of the recovery process following a radical prostatectomy.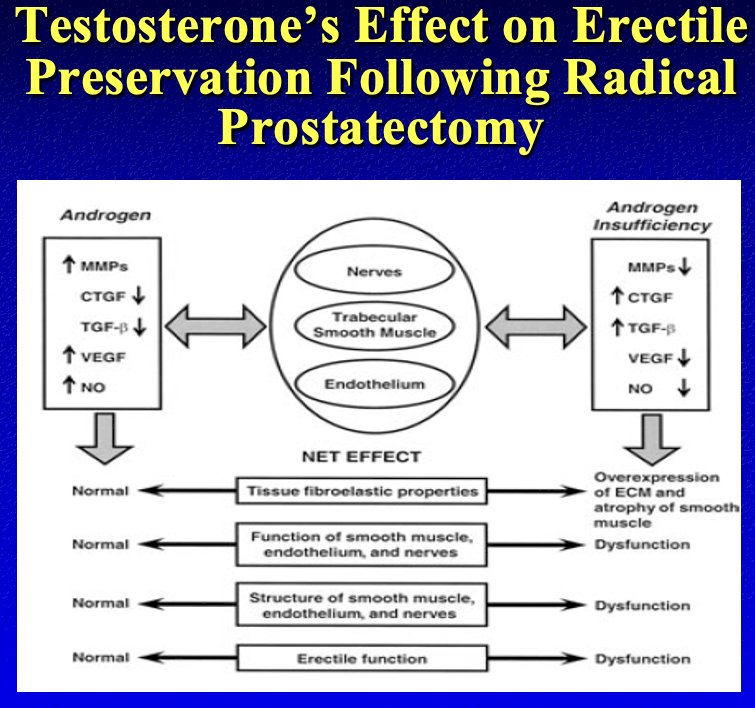
His concluding statements were as follows:
- There is currently no evidence that TRT promotes the initiation of prostate cancer in hypogonadal men
- Early data has not demonstrated any increased risk of prostate cancer recurrence or progression in men using TRT after prostate cancer
- Testosterone plays an important role in the recovery of erectile function following prostate cancer treatment
Presented by: Mohit Khera, MD, MBA, MPH, Professor, Scott Department of Urology at Baylor College of Medicine, Houston, TX
Written by: Rashid K. Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX between December 3rd and 6th, 2024
References:
- Kim M, Byun S, Hong SK. Testosterone Replacement Therapy in Men with Untreated or Treated Prostate Cancer: Do We Have Enough Evidences? World J Mens Health. 2021; 39(4):705-23.
- Pastuszak AW, Khanna A, Badhiwala N, et al. Testosterone Therapy after Radiation Therapy for Low, Intermediate and High Risk Prostate Cancer. J Urol. 2015; 194(5):1271-6.
- Morgentaler A, Traish AM. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol. 2009; 55(2):310-20.
- Khera M, Bhattacharya RK, Blick G, et al. Changes in prostate specific antigen in hypogonadal men after 12 months of testosterone replacement therapy: support for the prostate saturation theory. J Urol. 2011; 186(3):1005-11.
- Flores JM, Vertosick EA, Salter CA, et al. Testosterone Therapy in Men After Radical Prostatectomy for Organ-Confined, Low-Intermediate Prostate Cancer. J Urol. 2024.
- Loeb S, Folkvaljon Y, Damber JE, et al. Testosterone Replacement Therapy and Risk of Favorable and Aggressive Prostate Cancer. J Clin Oncol. 2017; 35(13):1430-6.
- Ahlering TE, Huynh L, Towe M, et al. Testosterone replacement therapy reduces biochemical recurrence after radical prostatectomy. BJU Int. 2020; 126(1):91-6.
- Testosterone for Penile Rehabilitation After Radical Prostatectomy. https://clinicaltrials.gov/study/NCT00848497. Accessed on December 5, 2024.
- Ferro M, Lucarelli G, Bruzzese D, et al. Low serum total testosterone level as a predictor of upstaging and upgrading in low-risk prostate cancer patients meeting the inclusion criteria for active surveillance. Oncotarget. 2017; 8(11):18424-34.
- Bhasin S, Travison TG, Pencina KM, et al. Prostate Safety Events During Testosterone Replacement Therapy in Men With Hypogonadism: A Randomized Clinical Trial. JAMA Netw Open. 2023; 6(12):e2348692.
- Linja MJ, Savinainen KJ, Saramaki OR, et al. Amplification and overexpression of AR gene in hormone-refractory PCa. Cancer Res. 2001;61:3550-3555.
- Attard G, Swennenhuis JF, Olmos D, et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant PCa. Cancer Res. 2009;69:2912-2918.
- Brown RS, Edwards J, Dogan A, et al. Amplification of the AR gene in bone metastases from hormone-refractory PCa. J Pathol. 2002;198:237-244.
- Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in PCa. N Engl J Med. 2014;371:1028-1038.
- Isaacs JT, D’Antonio JM, Chen S, et al. Adaptive auto-regulation of AR provides a paradigm shifting rationale for bipolar androgen therapy (BAT) for castrate resistant human PCa. Prostate. 2012;72:1491-1505.
- Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33-39.
- Isaacs JT, D’Antonio JM, Chen S, et al. Adaptive auto-regulation of AR provides a paradigm shifting rationale for bipolar androgen therapy (BAT) for castrate resistant human PCa. Prostate. 2012;72:1491-1505.
- Chatterjee P, Schweizer MT, Lucas JM, et al. Supraphysiological androgens suppress PCa growth through AR-mediated DNA damage. J Clin Invest. 2019;130:127613.
- Chuu CP, Hiipakka RA, Fukuchi J, Kokontis JM, Liao S. Androgen causes growth suppression and reversion of androgen-independent PCa xenografts to an androgen-stimulated phenotype in athymic mice. Cancer Res. 2005;65:2082-2084.
- Denmeade SR, Wang H, Agarwal N, et al. TRANSFORMER: A Randomized Phase II Study Comparing Bipolar Androgen Therapy Versus Enzalutamide in Asymptomatic Men With Castration-Resistant Metastatic Prostate Cancer. J Clin Oncol. 2021;39(12):1371-82.


