(UroToday.com) The 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX between December 3rd – 6th, 2024 was host to a prostate cancer poster session. Dr. Mark Sultan presented the results of an analysis demonstrating that the Decipher® Genomic Classifier (GC) score on the index biopsy is an independent predictor of clinical progression in active surveillance patients following adjustment for the initial MRI results.
Despite attempts to reduce prostate cancer overdiagnosis, approximately 30% of incident prostate cancer cases can be categorized as National Comprehensive Cancer Network (NCCN) low- or very low-risk tumors. Active surveillance is currently endorsed by numerous international guidelines to minimize the morbidity of overtreatment of such patients.1,2

However, the rate of progression to definitive therapy within five years of initiation of active surveillance ranges between 20% and 50%.3
This suggests that better early risk stratification is needed to identify those patients at increased risk of active surveillance ‘failure’. Accordingly, clinical tools such as multiparametric magnetic resonance imaging (mpMRI) and Decipher® GC are recommended for risk stratification in this setting.2
However, there is limited longitudinal evidence to support the value of tissue biomarker testing, independent of mpMRI findings, to improve the detection of clinically significant prostate cancer among active surveillance patients. As such, the primary study objective was to evaluate the associations between imaging (mpMRI) and genomic (Decipher® GC) risk stratification scores at prostate cancer diagnosis with the rate of progression to definitive therapy in active surveillance patients. The secondary objective was to evaluate whether combining mpMRI imaging with genomic testing adds predictive utility in this setting.
This was a retrospective analysis of consecutive active surveillance patients identified from biopsies submitted for Decipher® GC testing (Veracyte, Inc, San Francisco, CA) between December 2016 and December 2023. The following variables were evaluated at time of active surveillance initiation: serum prostate-specific antigen (PSA) level, the index biopsy Gleason Grade Group, NCCN risk group, mpMRI Prostate Imaging-Reporting and Data System (PI-RADS) classification score, and Decipher® GC score. The timing of treatment progression was collected, where applicable. The mpMRI imaging findings were dichotomized as either negative (PI-RADS 1–3) or positive (PI-RADS 4–5). The Decipher® score was risk categorized as low (<0.45), intermediate (0.45–0.6), or high (>0.6). The associations between the initial risk profiles and the odds of treatment progression following active surveillance initiation were assessed using a chi-square statistic. Kendall’s tau-b (τb) correlation coefficient evaluated the collinearity between the Decipher GC score (≥0.45) and mpMRI findings (PI-RADS 4-5 lesions). The associations between the mpMRI risk groupings and Decipher® GC risk score, each evaluated independently and then combined, with the rates of treatment progression were assessed using Cox proportional hazards regression modeling.
The baseline patient characteristics (n=338) are summarized below.
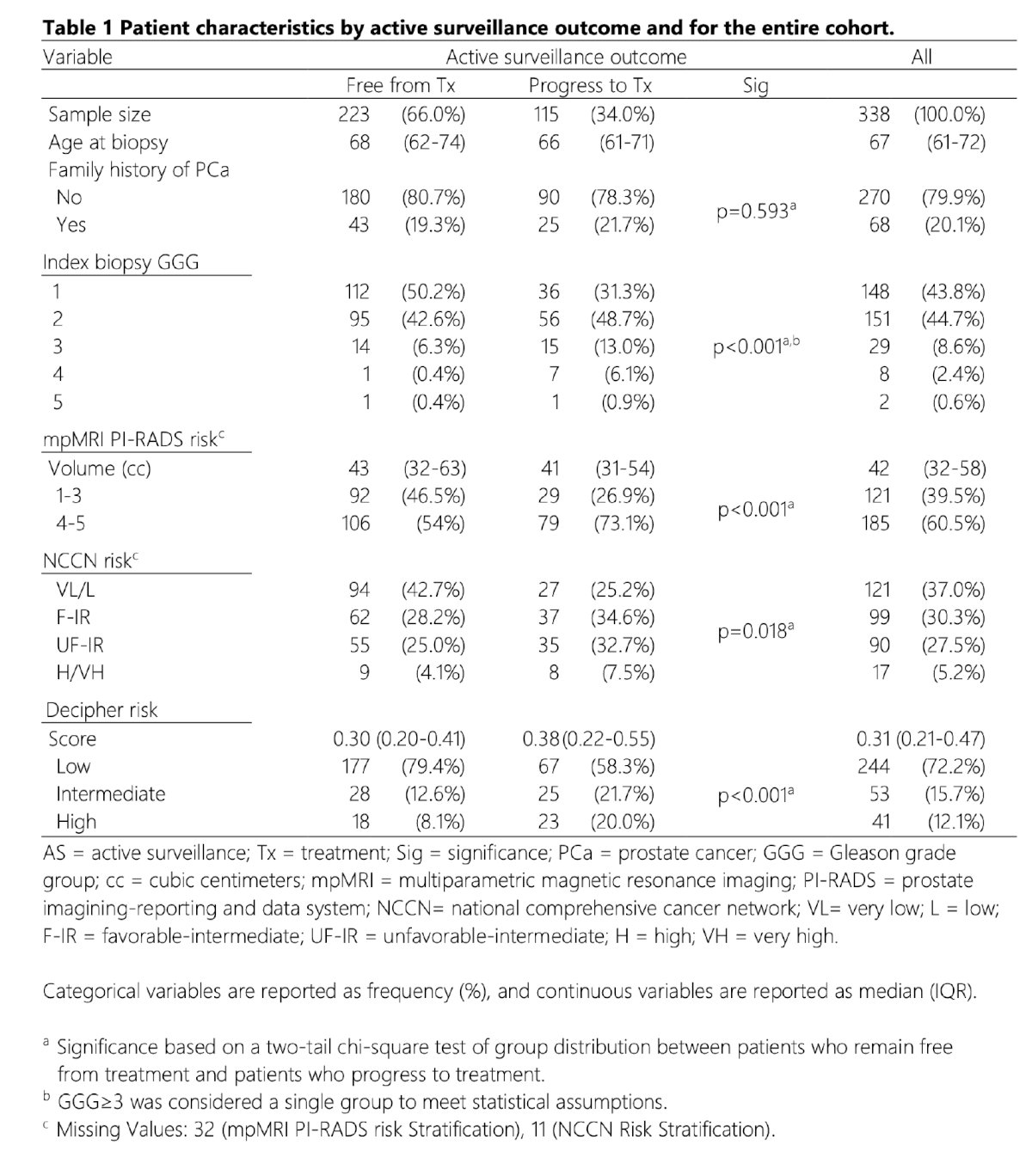
The mean serum PSA level was 7.4 ng/ml. Overall, 34% of patients progressed to definitive therapy during the median study follow-up of 26 (IQR: 16–40) months.
When the associations between each of the Decipher® GC score and mpMRI findings with the rates of treatment progression were independently evaluated, the study investigators demonstrated that a Decipher® GC score ≥0.45 generated a hazard ratio (HR) of 2.04 (95% CI: 1.39–2.99, p<0.001), whereas a PI-RADS 4-5 finding yielded an HR of 1.85 (95% CI: 1.21–2.81, p=0.004) for treatment progression.
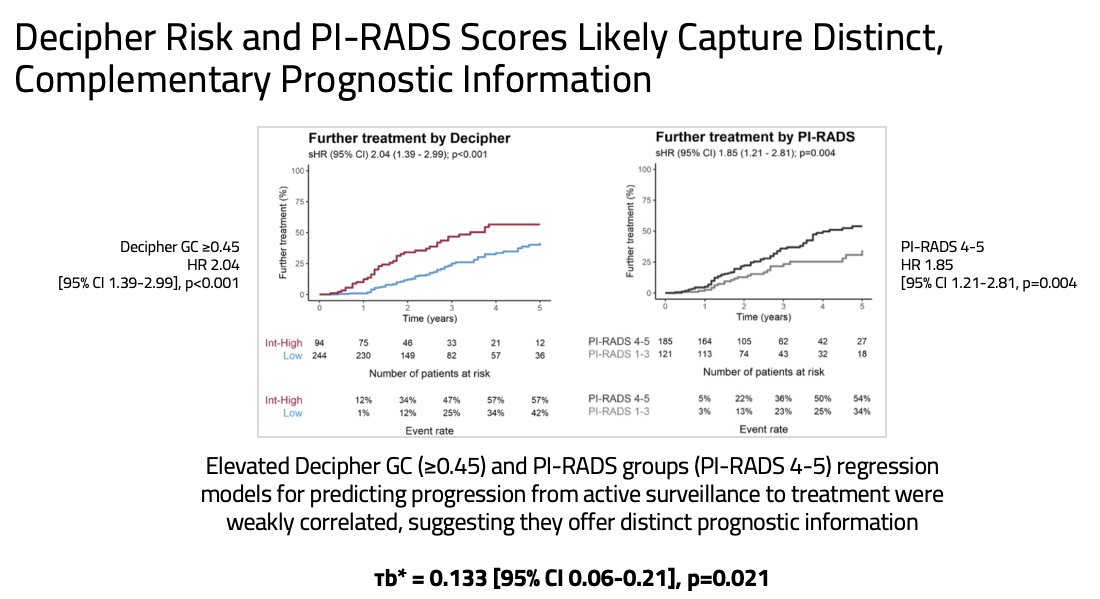
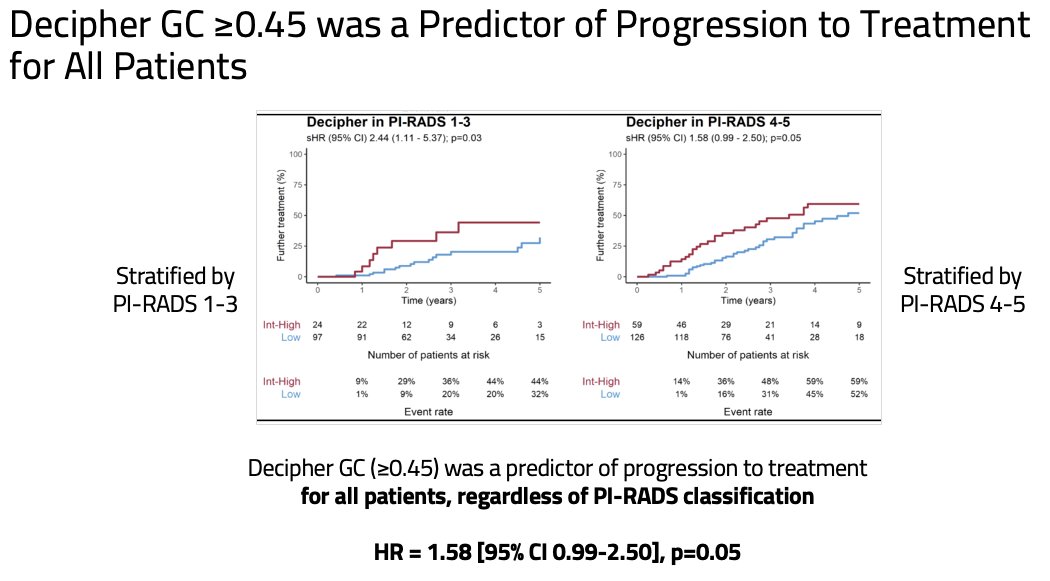
As genomic and imaging high-risk features were weakly correlated (τb = 0.133, p=0.021), the study investigators next evaluated the predictive utility of a Decipher® GC score ≥0.45, stratified by the mpMRI PI-RADS findings. They demonstrated that a Decipher® GC score ≥0.45 remained significantly predictive of rate of treatment progression for all patients, including the subset with PI-RADS 4-5 disease (HR: 1.58, 95% CI: 0.99–2.5, p=0.05).
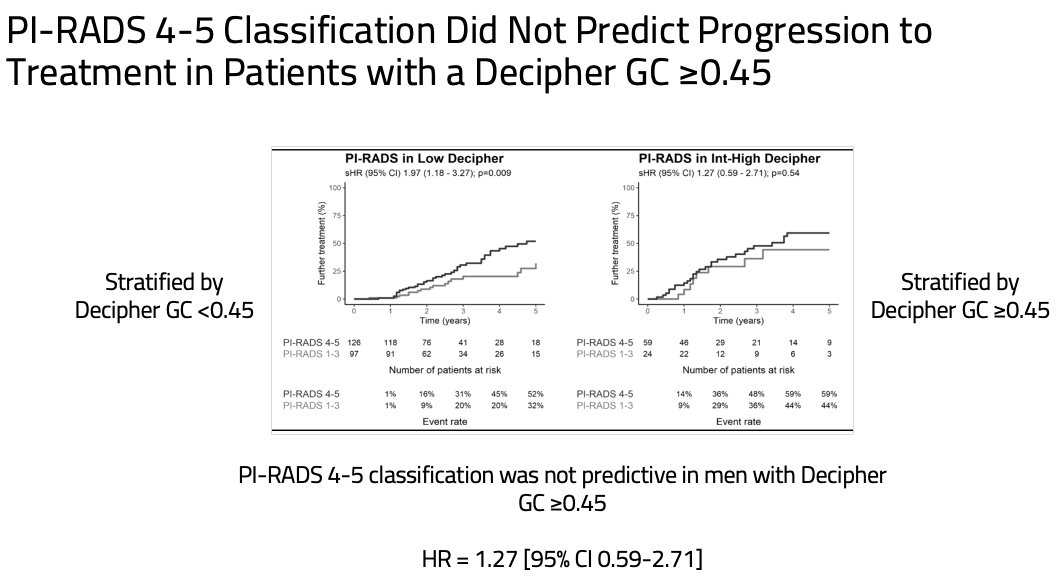
However, the reverse did not hold true – a PI-RADS 4-5 lesion finding on mpMRI was not predictive of treatment progression for men with a Decipher® GC score ≥0.45.
Based on these results, Dr. Sultan concluded as follows:
- Elevated Decipher® GC score (≥0.45) and mpMRI findings (PI-RADS 4-5 lesions) were associated with rates of treatment progression following initiation of active surveillance regimens
- The weak correlation between the two risk stratification tools suggests that both features capture distinct, complementary prognostic information and should ideally be used in combination.
- These results validate Decipher® GC score ≥0.45 as an independent predictor for progression to treatment for all active surveillance patients, irrespective of PI-RADS lesion classification
- mpMRI PI-RADS 4–5 classification was not predictive of treatment progression in patients with a Decipher® GC score ≥0.45
- These results suggest an individualized approach to active surveillance may be further enhanced using Decipher® GC testing, even for men with concerning mpMRI findings (e.g., PI-RADS 4-5)
Presented by: Mark Sultan, MD, Resident Physician, Department of Urology, The Mayo Clinic, Jacksonville, FL
Written by: Rashid K. Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, between the 3rd and 6th of December, 2024.
References:
- Schaeffer EM, Srinivas S, Adra N, et al. NCCN Guidelines® Insights: Prostate Cancer, Version 3.2024. J Natl Compr Canc Netw. 2024; 22(3):130-50.
- Eastham JA, Auffenberg GB, Barocas DA, et al. Clinically localized prostate cancer: AUA/ASTRO guideline, part I: introduction, risk assessment, staging, and risk-based management. J Urol. 2022; 208(1):10-8.
- Shill DK, Roobol MJ, Ehdaie B, Vickers AJ, Carlsson SV. Active surveillance for prostate cancer. Transl Androl Urol. 2021; 10(6):2809-19.


