(UroToday.com) At the 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX between December 3rd – 6th, 2024, Dr. Seth Lerner received the prestigious Huggins Medal award from the SUO committee.
Dr. Lerner began by acknowledging Dr. Charles Huggins whose ‘serendipitous’ work studying phosphate metabolism at the University of Chicago led to interest in steroid endocrinology and the finding that spontaneous prostate tumors shrink with an orchiectomy. His remarks about team science have long resonated, with Dr. Huggins quoted as saying: “I was never alone in my studies in which one or two students always participated as colleagues. It is a privilege to thank the scores of young men and women who sustained our work."
He won the Nobel Prize in Physiology or Medicine in 1966 for his discoveries concerning hormonal treatment of prostate cancer, and that cancer can be abrogated by orchiectomy or by adding female sex hormones.
Following his recognition of Dr. Huggins’ contributions to our field, he thanked his family for their continuous support over the past four decades, acknowledging that none of this would have been possible without their unwavering support throughout.
Next, he moved on to thanking his urology mentors – at Virginia Mason Clinic, where he completed his early general surgery training, at Baylor College of Medicine, where he completed his urology residency training, and at The University of Southern California, where he completed a two-year oncology and reconstructive fellowship under the guidance of Drs. Donald Skinner and Peter Jones.
One of Dr. Lerner’s main urologic inspirations has long been Dr. Don Griffith, a urologic stone expert, who was one of the pioneers in the early development and adoption of extra-corporeal shock wave lithotripsy (ESWL) and an early adopter of percutaneous nephrolithotomy (PCNL), and who mentored Dr. Lerner during his 1st year of urologic residency training.
What is team science? It is a collaborative effort to address a scientific challenge that leverages the strengths and expertise of professionals, oftentimes trained in different fields. It typically relies on coordinated teams of investigators with diverse skills and knowledge that may be especially helpful for tackling complex scientific and societal problems.
One of Dr. Lerner’s earliest experiences in team science involved building the Bladder Cancer Research Consortium in 2003 in collaboration with physician colleagues from Baylor, Johns Hopkins, and UT Southwestern. This consortium included 958 patients who underwent a radical cystectomy plus a pelvic lymph node dissection with curative intent and had complete pathologic staging available. Importantly, this group employed an expert biostatistician (Pierre Karakiewicz), without whom none of this would have been possible. Numerous seminal studies ensued from this patient cohort that allowed us to better understand the clinicopathologic underpinnings of muscle-invasive bladder cancer, and multiple nomograms were developed to predict advanced stage disease, recurrence, and survival outcomes. For example, a study from this consortium helped establish lymphovascular invasion as an adverse prognostic feature in bladder cancer patients.
Some of the early team science lessons learned by Dr. Lerner from this early consortium work included the following:
- Engage all members of the team
- Identify most, if not all, projects at outset
- Each team picks what they want to work on
- Establish rules of engagement at the start
- Authorship is key
- Data ownership
- Support and create opportunities for residents and fellows
- Work with a great biostatistician
The seeds of the genesis of the The Cancer Genome Atlas (TCGA) MIBC project were planted in Lund, Sweden where Dr. Lerner was a guest of Drs. Wiking Manson and Mattias Hoglund, who inspired him to embark on this journey. Dr. Lerner noted, however, that none of this would have been possible without Dr. Richard Gibbs, who authored the 1st TCGA marker paper for glioblastoma multiforme (GMB), was convinced that this was similarly feasible for bladder cancer, and supported Dr. Lerner throughout this journey.
Summarized below are the team members of the TCGA MIBC Working Group, which highlights the truly unique team nature behind this collaborative scientific endeavor.
He summarized the TCGA pipeline, illustrated below. 722 tissue samples were obtained from 36 tissue source sites and were subsequently analyzed as follows: They subsequently performed a comprehensive molecular characterization of these urothelial bladder carcinoma tissue specimens, which were obtained from patients with cT1-4aN0-3M0-1 disease and who had a mean follow-up of 26 months.1,2
They subsequently performed a comprehensive molecular characterization of these urothelial bladder carcinoma tissue specimens, which were obtained from patients with cT1-4aN0-3M0-1 disease and who had a mean follow-up of 26 months.1,2 
What is the impact of TCGA for MIBC? This is a multi-platform integrated genomic analysis with data that is publicly available for researchers worldwide (cbioportal.org). A brief internet search demonstrates the impact of the TCGA cohort, with almost 1,400 studies having ensued from this cohort.
Seminal work from this cohort has led to the discovery of bladder cancer molecular subtypes: luminal papillary, luminal infiltrated, luminal, basal squamous, and neuronal.
These molecular subtypes have been demonstrated to be associated with different survival outcomes, which is of utmost clinical importance.
This work has since been validated in numerous cohorts, including the IMvigor 210 cohort of patients with platinum-refractory or cisplatin-ineligible urothelial carcinoma who were treated with atezolizumab.2
In 2015, in collaboration with colleagues from Madrid, including Dr. Paco Real, Dr. Lerner and colleagues helped establish the now well-established, consensus classification of bladder cancer molecular subtypes, summarized in the figure below:
This work has again influenced our understanding of bladder cancer molecular subtypes and these phenotypes have been correlated with survival outcomes:
Dr. Lerner and colleagues next demonstrated that the molecular subtypes may be predictive of treatment response: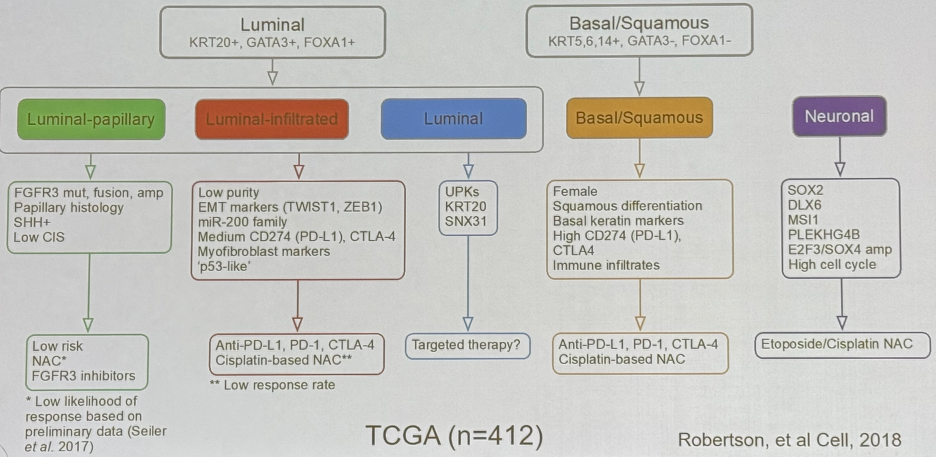
This work has led to the design of the SUBTYP trial, which is a multi-arm platform trial comparing standard of care therapy to subtype-directed therapy in muscle invasive bladder cancer patients.
Dr. Lerner next reflected on his experience with the Southwest Oncology Group (SWOG), NCI National Clinical Trials Network (NCTN), and Cancer Therapy Evaluation Program (CTEP). When Dr. Lerner first started at Baylor in 1992, he began attending the biannual SWOG meetings, which were a great opportunity to learn from giants in this field and opened opportunities to enrol clinical trial patients at Baylor. This subsequently led to leadership and mentoring opportunities, including co-chairing the testis, penile, adrenal, and rare diseases committee in 2003 and the local bladder cancer committee from 2009 to 2023. Numerous clinical trials were opened in collaboration with SWOG (S1011, S1314, S1602, S1605, S1806, S1937, S2011) and over 2,600 patients have since been enrolled.
Dr. Lerner later became the founding co-chair of the CTEP bladder cancer task force and the co-chair of the GU steering committee. Most recently, Dr. Lerner fulfilled a lifelong ambition of his by becoming the chair of the SWOG GU committee in July 2023.
One of the important lessons that Dr. Lerner has learned over the years is the importance of validation studies. The coexpression extrapolation (COXEN) algorithm-generated gene expression model (GEM) score was developed to help predict which patients undergoing a radical cystectomy may benefit from neoadjuvant chemotherapy. Despite initial promising results, two validation studies have since failed to definitively confirm its predictive utility in this setting.4,5
However, one of the crowning achievements from his work with the SWOG committee has been the SWOG S1011 trial that was recently published in The New England Journal of Medicine.6 This trial has taken more than a decade to complete, with the first patient registered in August 2011 and the last registered in March 2017. The total time to report out took ~13 years. This trial failed to demonstrate a survival benefit to extended pelvic lymphadenectomy, compared to standard template lymphadenectomy, at the cost of increased 30- and 90-day mortalities (2.7% versus 0.3% and 6.5% versus 2.4%, respectively).
What’s next for the SWOG GU committee? Dr. Lerner highlighted the plethora of ongoing trials led by this committee:
- S1802 – Oligometastatic prostate cancer – principal investigator (PI) Brian Chapin
- S1931 – PROBE - CRN + 10/TKI - PI Hyung Kim
- S1937 – 2nd/3rd line metastatic bladder cancer - PI Sarmad Sadeghi
- S2200 – PAPMET2 - PI Ben Maughan
- S2210 – BRCA Germline variant high risk PCA/RRP - PI Heather Cheng
- S2312 – Aggressive variant mCRPC - PI Paul Corn
- S2427 – NAC + RT/IO - PI Leslie Ballas
- S2419 – BioFront - Microbiome and immunotherapy in metastatic RCCA - PI Pedro Barata
- CCTG/SWOG - Triple Switch - mCSPC PI - Sasha Sokolova
What were the lessons learned from his time at SWOG and NCTN?
- Engage and stay engaged - your turn will come
- We should answer important scientific questions
- The mission is practice changing, large phase II and III trials
- The essence of team science
- SWOG network involves over 350 institutions
- North American and Latin America
- Rely on best-in-class biostatisticians and operations experts
- Multi-disciplinary engagement throughout the concept/protocol/trial management process is key
- Patient advocates and CORP/community physicians should be engaged throughout
- There is a treasure chest of mentoring and leadership opportunities available out there
Dr. Lerner touched upon his experiences with the FDA, AUA, and SUO to establish guidance for conducting single arm registrational trials for agents in the BCG-unresponsive NMIBC. Prior to 2014-16, the last agent to be approved for these patients was valrubicin, which has modest activity in this setting, approved in 1998. Since then, and supported by numerous leaders in this field, including Dr. Colin Dinney at MD Anderson, three agents have been approved for BCG-unresponsive NMIBC (2020: pembrolizumab; 2022: nadofaragene; 2024: N803).
To conclude, Dr. Lerner emphasized: “Don’t forget to have fun along the way”.
Presented by: Seth Lerner, MD, FACS, Professor of Urology, Beth and Dave Swalm Chair in Urologic Oncology, Vice-Chair for Faculty Affairs, Scott Department of Urology, Baylor College of Medicine, Houston, TX
Written by: Rashid K. Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX between December 3rd and 6th, 2024
References:- Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell. 2017; 171(3):540-56.e25.
- Kim J, Kwiatkowski D, McConkey DJ, et al. The Cancer Genome Atlas Expression Subtypes Stratify Response to Checkpoint Inhibition in Advanced Urothelial Cancer and Identify a Subset of Patients with High Survival Probability. Eur Urol. 2019; 75(6):961-4.
- Kamoun A, de Reynies A, Allory Y, et al. A Consensus Molecular Classification of Muscle-invasive Bladder Cancer. Eur Urol. 2020; 77(4):420-33.
- Flaig TW, Tangen CM, Daneshmand S, et al. A Randomized Phase II Study of Coexpression Extrapolation (COXEN) with Neoadjuvant Chemotherapy for Bladder Cancer (SWOG S1314; NCT02177695). Clin Cancer Res. 2021; 27(9):2435-41.
- Flaig TW, Tangen CM, Daneshmand S, et al. Long-term Outcomes from a Phase 2 Study of Neoadjuvant Chemotherapy for Muscle-invasive Bladder Cancer (SWOG S1314; NCT02177695). Eur Urol. 2023; 84(3):341-7.
- Lerner SP, Tangen C, Svatek RS, et al. Standard or Extended Lymphadenectomy for Muscle-Invasive Bladder Cancer. N Engl J Med. 2024; 391(13):1206-16.


