(UroToday.com) The 2024 SUO annual meeting included a session on the current controversy of seminoma, featuring a State of the Art presentation by Dr. Richard Matulewicz discussing controversies in the surgical management of seminoma and ideal patient selection for primary RPLND. Dr. Matulewicz started his presentation by reviewing the clinical staging of nodal disease, in particular highlighting that “bulky disease” is retroperitoneal lymph nodes > 3 cm:

There are ~10,000 cases of testicular germ cell tumor in the United States each year, with ~60% comprising pure seminoma. By stage, this represents 80%+ CSI seminoma and ~15% are CSIIAB seminoma. Approximately ~15-20% of CSI seminoma will relapse, most often (94%) in the retroperitoneum alone. Thus 7% (60% x 80% x 15%) + 8% (60% x 15%) = 15% of all testicular germ cell tumors are cN+. Long term survival is the expectation, given that 98-99% are early stage disease.
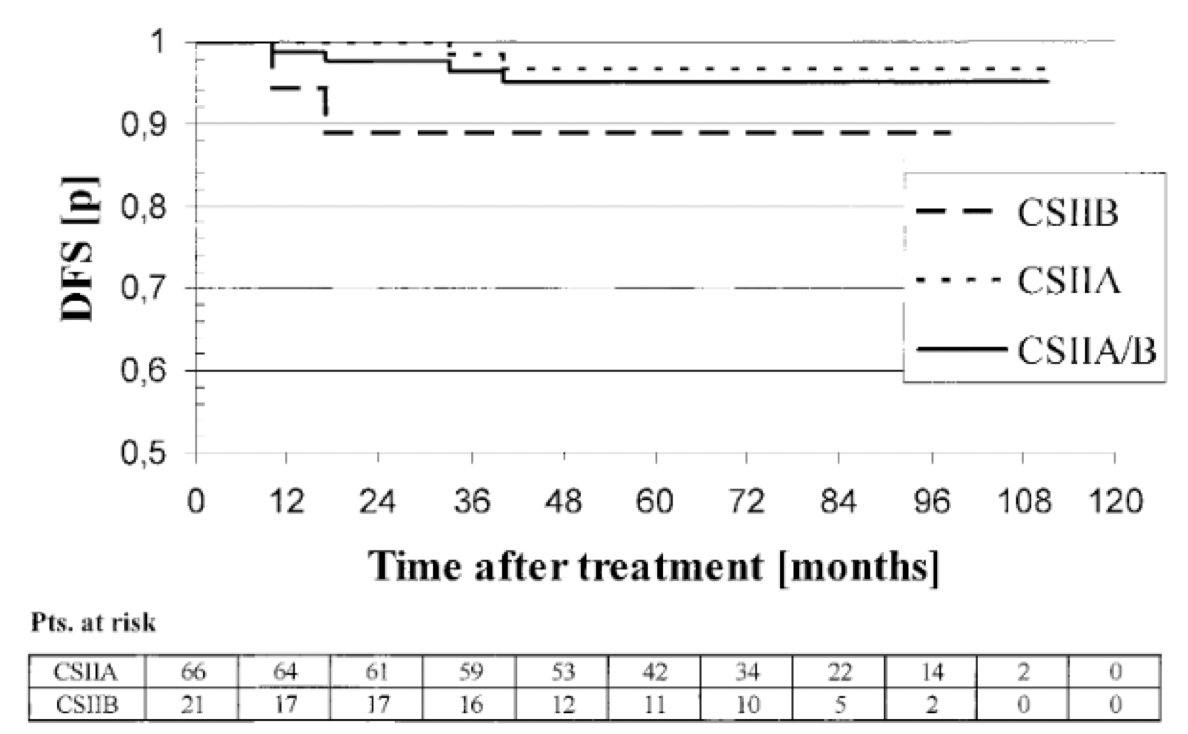
To date, there are no randomized trials comparing radiotherapy to chemotherapy for CSII pure seminoma. Dr. Matulewicz highlighted two studies which are most applicable, the first is a German radiotherapy study looking at outcomes of 87 patients with CSIIA or CSIIB disease treated with 30 or 36 Gy (hockey stick template) depending on stage.1 In this study, long-term disease free survival was 95% for IIA and 89% for IIB, with disease specific survival nearly 100%:
The European GETUG S99 chemotherapy study of patients with metastatic seminoma used a risk adapted strategy, with the patients in the good risk cohort receiving EP x4 cycles.2 Excellent outcomes are also reported, with a 3 year progression free survival of 93% and overall survival approaching 100%, with one death secondary to suicide (good risk – orange bar):
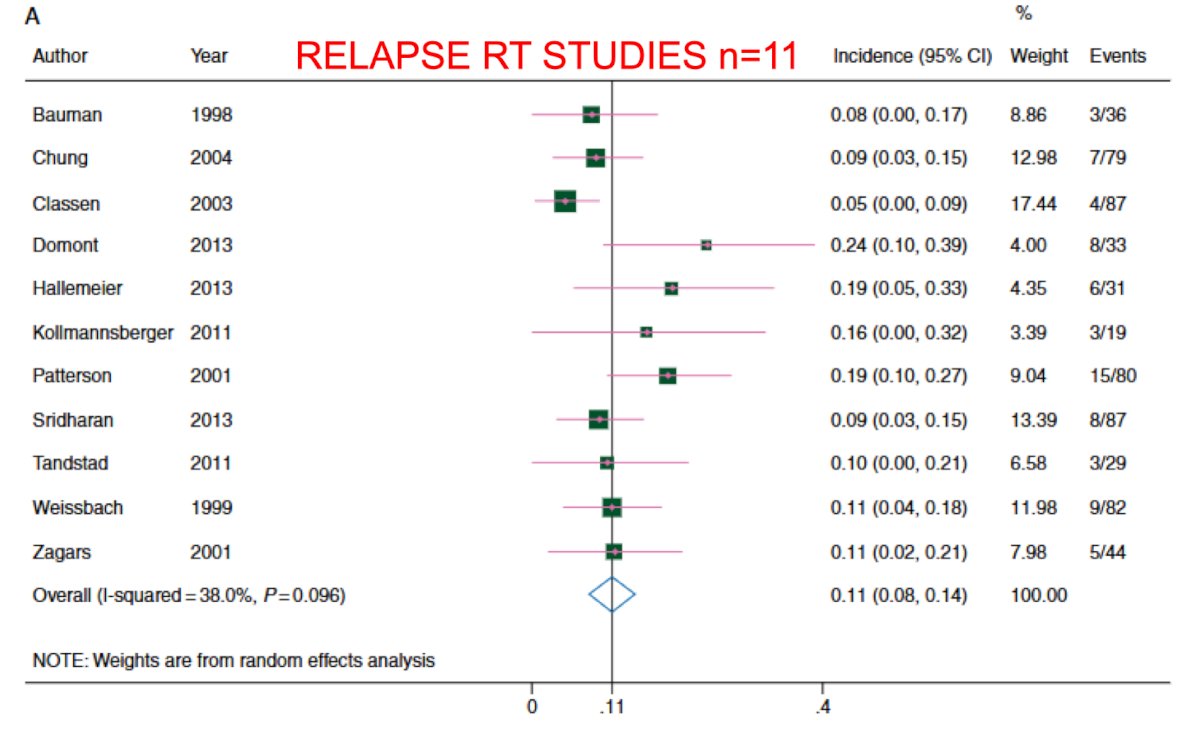
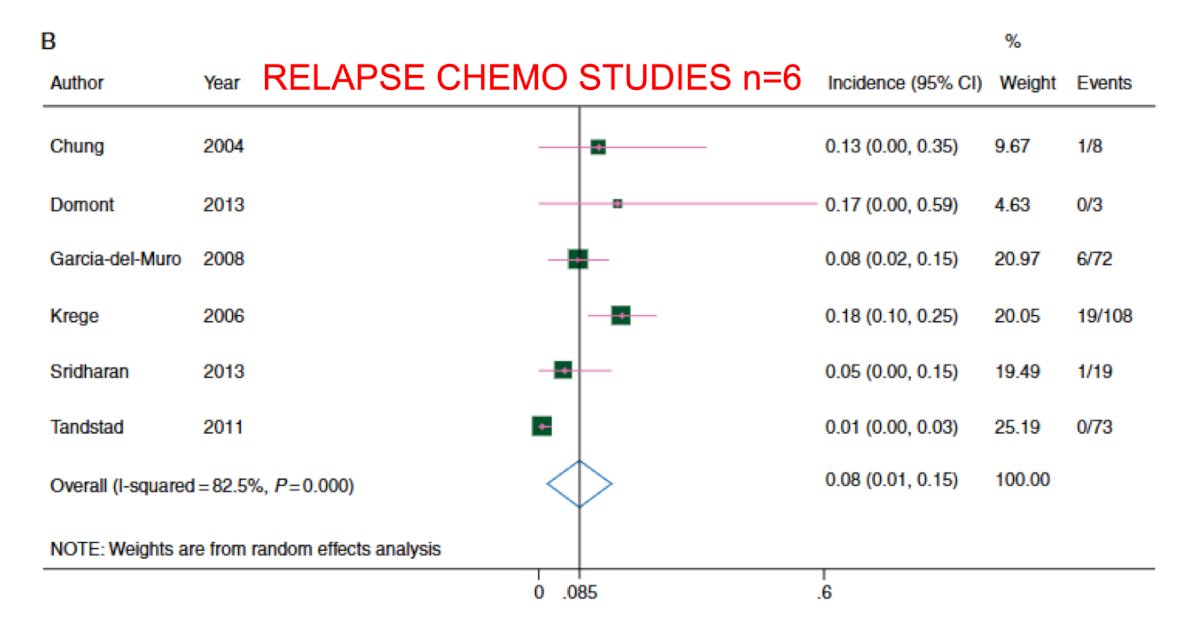
Again, although there are no randomized trials comparing radiotherapy to chemotherapy for CSII pure seminoma, there has been a high quality meta-analysis that compared outcomes in 11 radiotherapy studies and 6 chemotherapy studies.3 For radiotherapy, the 6 year recurrence free survival rate was ~95%, and for chemotherapy the 3 year recurrence free survival rate was 93%:
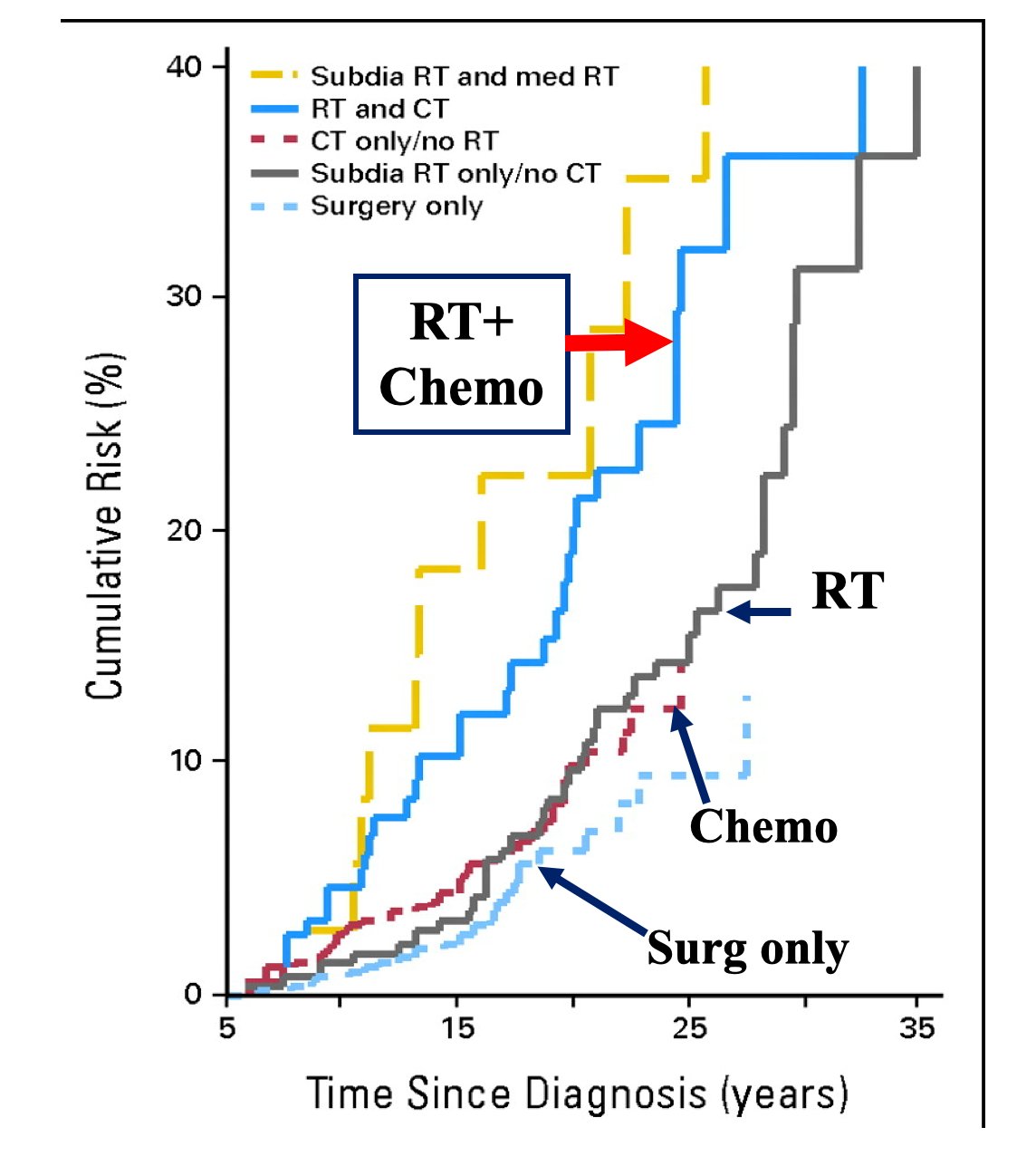
Importantly, the chemotherapy related risks of cardiovascular toxicity and secondary cancers are equivalent to the risks of long-term smoking: there is a ~2x increased risk for cardiovascular toxicity and secondary cancer, which is dose dependent, and often fatal within 5 years4:
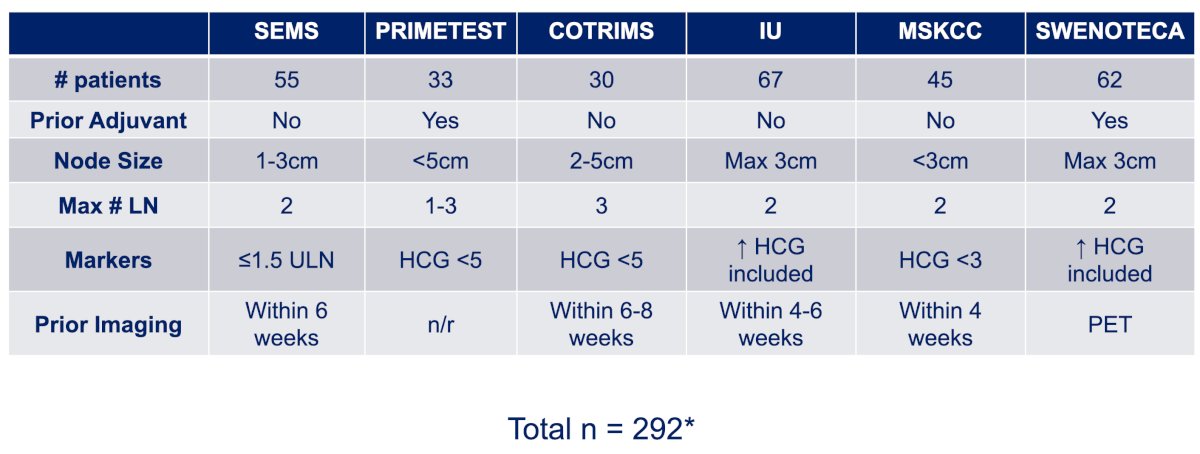
In the last several years, there has been emerging data to support the use of primary RPLND for the treatment of pure seminoma with low volume metastases to the retroperitoneum. The first was a German study (PRIMETEST5), which was followed by the SEMS trial,6 both prospective Phase II studies. After this, additional institutional series were published as well as the COTRIMS trial7 and a population based approach from the SWENOTECA group.8
Despite these studies, there are still many unanswered questions, and in Dr. Matulewicz’s opinion, room for continued optimization and improvement. However, the results of these studies have primary RPLND now included in both the NCCN and AUA guideline recommendations for the management of retroperitoneal only metastatic pure seminoma for patients with <3 cm lymph nodes.
Dr. Matulewicz notes that patient selection is the key to good surgical outcomes. Improvements in patient selection previously demonstrated high chance of cure with surgery alone in NSGCT by (i) avoiding elevated serum tumor markers, and (ii) avoiding CSIIB/CSIII disease, with the guiding principle that we do not operate on patients with a high likelihood of occult disease. But, seminoma is not NSGCT. Seminoma is a nodal disease with predictable patterns of metastases and Halsteadian spread to the lymph nodes. Radiation studies suggest that 0.6% of patients with CSI seminoma relapse in the chest with PA/DL radiation, 1.7% of CSI patients have pelvic relapse after PA radiation, and 3.2% of CSIIAB patients have extra-retroperitoneal relapse after DL radiation. In surveillance studies, 94% of CSI have retroperitoneal relapse only, and systemic/visceral/non-nodal relapses are extraordinarily rare. From a surgical perspective, the inclusion criteria from the published studies are as follows:
The first important question is whether there are variations in outcomes with how patients initially present. Specifically, is there a difference in relapsed versus de novo retroperitoneal nodal disease, or is it strictly based on maximum nodal size?
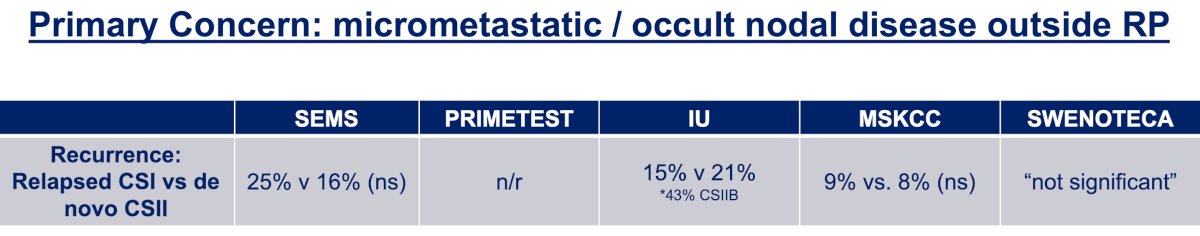
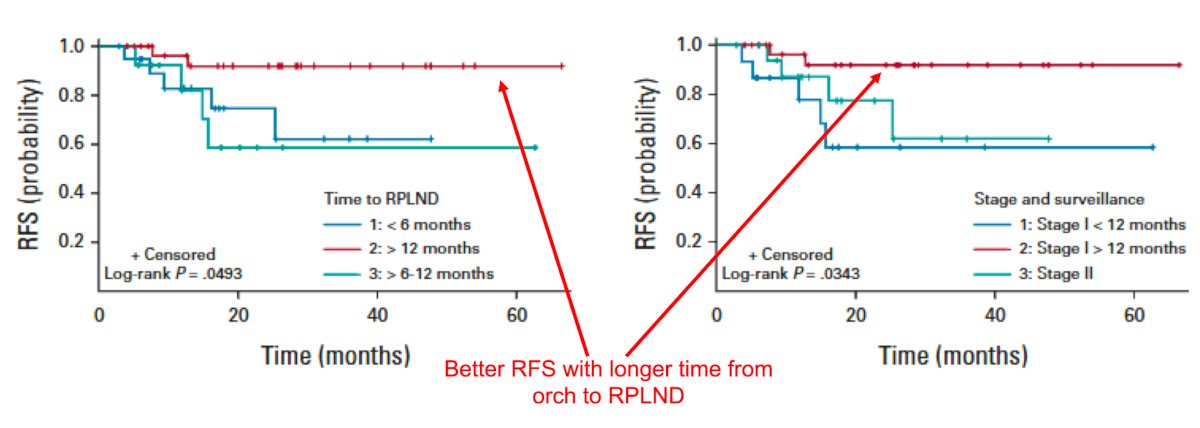
Dr. Matulewicz notes that on the surface, there does not appear to be significantly different relapse rates for those who present with de novo CSII disease versus those patients who relapse from CSI disease. However, the raw relapse rates may not tell the whole story, given that adjuvant therapy was not uniformly avoided and this does not take into account pathologic staging which could be different from clinical staging within studies. He notes that the best available data is from the Indiana series, demonstrating that the time from orchiectomy to RPLND, particularly for those with >1 year between operations, was associated with a lower risk of recurrence than those with <1 year, regardless of whether they had relapsed CSI or de novo CSII.9 Taken together, this suggests that there is a biological continuum to this process that should be acknowledged and respected:
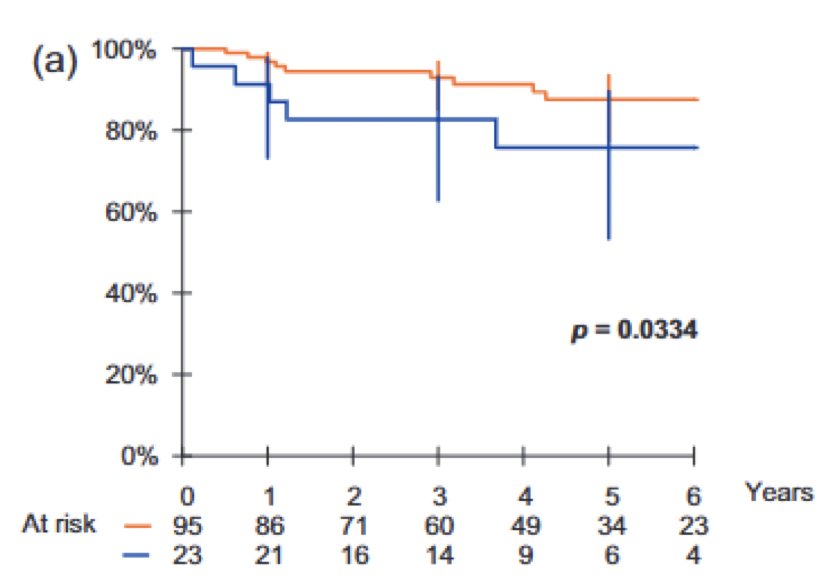
This has been similarly reported in patients receiving radiotherapy, where based on retrospective data, 5-year recurrence free survival was higher (92%) in relapsed patients and lower (73%) in de novo patients:
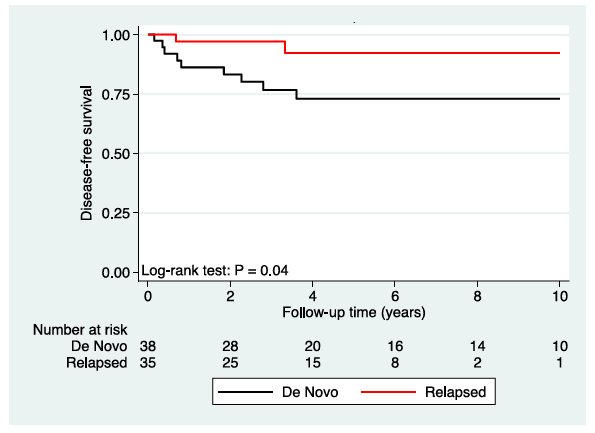
Similarly, the SAKK 01/10 trial performed a prespecified post hoc analysis of de novo versus relapsed CSII patients and found that all recurrences were in the de novo arm (100% recurrence free survival at 3 years in relapsed CSII versus 90.3% in de novo CSII).
Other considerations/unanswered questions include:
- Multiplicity of lymph nodes? This has not yet been thoroughly explored to date, but ideally, 2 enlarged lymph nodes are likely the maximum
- Prior HGC elevation? What are the implications of slightly elevated HCG/LDH prior to orchiectomy? Or prior to RPLND? This is still unclear, but trivial elevations probably are inconsequential prior to RPLND, however, the data is limited due to infrequent relapses and a lack of pooled analyses
- How do we approach suspected/occult pelvic lymphadenopathy? Avoid RPLND or perform adjunctive pelvic lymph node dissection?
- What do we do with prior inguinal surgery? Does this change the lymphatic drainage and metastatic patterns? It is unclear given the low frequency of events within studies, but there is no difference in the MSKCC series
With regards to pathologic upstaging, in the MSKCC series, 44% of patients were upstaged from cN1 to pN2-3 (4% pN3) and 7 % were downstaged from cN1 to pN0-1. In the SEMS trial, 44% of patients were upstaged, including 5% to pN3 (1 patient had a 12 cm lymph node), whereas 20% were downstaged. Dr. Matulewicz notes that this is likely a function of imaging gaps and likely “matted” nodes, and significant upstaging to cN3 is hopefully avoidable in most cases. Downstaging is mostly a function of pN0 rates, with variation between studies with the highest pN0 rate (16%) coming from the SEMS trial and smaller proportions of patients in some of the reported series. Of note, Indiana/MSKCC/SWENOTECA either have policies or tend to repeat imaging in equivocal nodes whereas this may not have been as uniformly practiced in other studies:

The most common situation where pN0 pathology was found was in smaller, equivocal nodes between 1-1.5cm, and in the SEMS trial, 30% of patients with nodes of this size were pN0. To date, there have been no recurrences reported for pN0 in the published literature.
What to do with lung nodules is a vexing problem and not uncommon in daily clinical practice. Dr. Matulewicz notes that this is much more concerning in NSGCT, especially those with choriocarcinoma or embryonal components, but in seminoma, this is less concerning. Approximately ¼ of all patients with presumed CSI seminoma have at least 1 sub-centimeter lung nodule seen on initial staging imaging, with no relapses seen among these patients whether they continued surveillance or were treated. Solitary lung recurrences are exceedingly rare and there have been no reported lung only relapses in any published studies to date. The one lung recurrence in COTRIMS ends up being secondary to embryonal carcinoma.
How can we improve our ability to accurately stage people beyond conventional imaging and thresholds? Based on NCCN recommendations, imaging should be done within 4 weeks of surgery. In the SEMS trial, the relapse rate for imaging > 4 weeks was 54% versus 12% for imaging < 4 weeks prior to RPLND (p = 0.004). It is important to review our own imaging, paying attention to change in lymph node size, multifocality, and the retrocrural space and pelvis. Short interval repeat imaging is likely safe given the kinetics of seminoma and the predilection for nodal spread of disease. In the Indiana series, 7 patients with 1-1.5 cm nodes surveilled between 3-12 months before RPLND all progressed to true CSII, although none recurred after treatment. Moreover, waiting is unlikely to eliminate surgery as a treatment option, but if there is significant disease progression, chemotherapy is better in these situations. Ultimately, we should aim to repeat a CT of the chest/abdomen/pelvis within 2-4 weeks of surgery.
Currently, there is no role for PET imaging, as there is insufficient data. Known limitations of PET imaging include (i) avidity present in inflammatory nodes, which is common with seminoma, and (ii) non-specific uptake being common in the retroperitoneum, including the ureter and ganglion both of which are false positives. Furthermore, there are unclear diagnostic parameters, with prior data mostly for post chemotherapy masses (very poor PPV, but a reasonably good NPV), and not generalizable to pre-treatment disease.
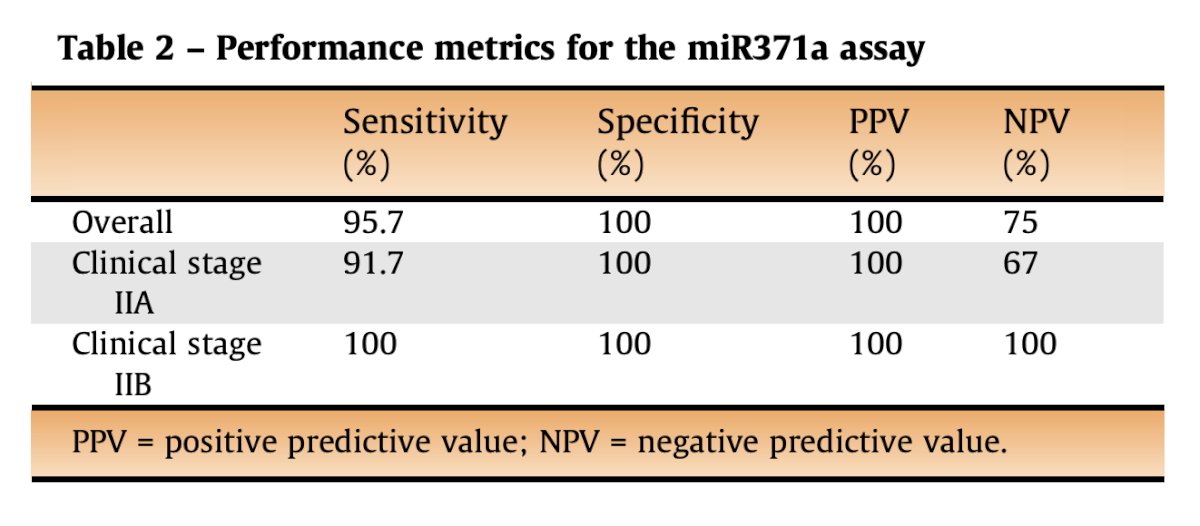
miRNA remains a topic of debate but shows promise in helping with diagnostic staging. Only a few reports have been published, mostly among mixed cohorts but the assay appears to be highly specific in most situations11:
Though anecdotally, at MSKCC, Dr. Matulewicz notes they have had issues with low volume disease in both the orchiectomy and primary RPLND setting.
Dr. Matulewicz also highlighted general principles for primary RPLND in metastatic seminoma:
- Definitive pre-operative staging and kinetics of seminoma allow for:
- Repeat short interval imaging (6 weeks)
- Will limit pN0 dissections
- Allows us to understand potential disruptions to predictability, such as prior inguinal surgery or prior adjuvant chemotherapy
- Operate with curative intent:
- Avoid high volume pathology reports (pN3, high volume positive lymph nodes), thus there is no role for “debulking”
- Check imaging for occult extra-retroperitoneal disease (ie. pelvis, retrocrural)
- Lung nodules are likely red herrings
- Aim for complete dissection in order to avoid surgical failures
It is important to highlight that “patient selection is your silent partner” – Dr. Whitmore:
- The time from orchiectomy to RPLND is likely important, but still unclear how prognostic this is on an individual level and how to approach clinically given the excellent outcomes for most patients
- Short interval repeat imaging in equivocal lymphadenopathy can help reduce pN0 rates and is unlikely to result in significant progression
- Up to date imaging should be obtained within 4 weeks of surgery
- PET may be potentially helpful in adjudicating equivocal masses, but false positive/negative rates are a concern and PET is not currently recommended
- miRNA is highly specific, but the clinical role of miRNA or ctDNA is still to be determined
Dr. Matulewicz concluded his presentation with several future directions:
- There is potential for expanding indications for primary RPLDN, but limiting over/under treatment with any modality, comparative health related quality of life implications, and improved early detection of relapse are key
- miRNA for CSI on active surveillance (SWOG 1823 and COG/AGCT1531)
- MAGESTIC Trial: miRNA for surgical decision making (all early stage GCT)
- PRESTIGE study: comparative study of RPLND versus chemoradiation in health related quality of life (mostly early stage GCT)
- UK “OTIS” trial
- ctDNA
Presented by: Richard Matulewicz, MD, MSCI, MS, Memorial Sloan Kettering Cancer Center, New York, NY
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Society of Urologic Oncology (SUO) Annual Meeting, Dallas, TX, Tues, Dec 3 – Fri, Dec 6, 2024.
References:
- Classen J, Schmidberger H, Meisner C, et al. Radiotherapy for stages IIA/B testicular seminoma: Final report of a prospective multicenter clinical trial. J Clin Oncol. 2003 Mar 15;21(6):1101-1106.
- Fizazi K, Delva R, Caty A, et al. A risk-adapted study of cisplatin and etoposide, with or without ifosfamide, in patients with metastatic seminoma: Results of the GETUG S99 multicenter prospective study. Eur Urol. 2014 Feb;65(2):381-386.
- Giannatempo P, Greco T, Mariana L, et al. Radiotherapy or chemotherapy for clinical stage IIA and IIB seminoma: A systematic review and meta-analysis of patient outcomes. Ann Oncol. 2015 Apr;26(4):657-668.
- Van den Belt-Dusebout AW, de Wit R, Gietema JA, et al. Treatment-specific risks of second malignancies and cardiovascular disease in 5-year survivors of testicular cancer. J Clin Oncol. 2007 Oct 1;25(28):4370-4378.
- Hiester A, Che Y, Lusch A, et al. Phase 2 Single-arm Trial of Primary Retroperitoneal Lymph Node Dissection in Patients with Seminomatous Testicular Germ Cell Tumors with Clinical Stage IIA/B (PRIMETEST). Eur Urol. 2023; Jul;84(1):25-31.
- Daneshmand S, Cary C, Masterson T, et al. Surgery in early metastatic seminoma: A phase II trial of retroperitoneal lymph node dissection for testicular seminoma with limited retroperitoneal lymphadenopathy. J Clin Oncol. 2023 Jun 1;41(16):3009-3018.
- Heidenreich A, Paffenholz P, Hartmann F, et al. Retroperitoneal lymph node dissection in clinical stage IIA/B metastatic seminoma: Results of the COlonge Trial of Retroperitoneal Lymphadenectomy In Metastatic Seminoma (COTRIMS). Eur Urol Oncol. 2024 Feb;7(1):122-127.
- Thor A, Negaard HFS, Bergdahl AG, et al. Primary retroperitoneal lymph node dissection as treatment for low-volume metastatic seminoma in a population-based cohort: The Swedish Norwegian Testicular Cancer Group Experience. Eur Urol Open Sci. 2024 Jun 11:65:13-19.
- Tachibana I, Alabd A, Tong Y, et al. Primary retroperitoneal lymph node dissection for stage II seminoma: Is surgery the new path forward. J Clin Oncol. 2023 Aug 10;41(23):3930-3938.
- Rosen DB, Ghosh A, Niemierko A, et al. Clinical outcomes of de novo versus relapsed early metastatic testicular seminoma treated with contemporary radiation therapy. Int J Radiat Oncol Biol Phys. 2024 Mar 1;118(3):706-711.
- Seelemeyer F, Pfister D, Pappesh R, et al. Evaluation of miRNA-371a-3p assay for predicting final histopathology in patients undergoing primary nerve-sparing retroperiteonal lymphadenectomy for stage IIA/B seminoma or nonseminoma. Eur Urol Oncol. 2024 Jun;7(3):319-322.


