(UroToday.com) The 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, TX was host to a session addressing current controversies in the management of seminoma patients. Dr. Timothy Masterson discussed strategies for retroperitoneal lymph node dissections (RPLND) in metastatic testicular seminoma.
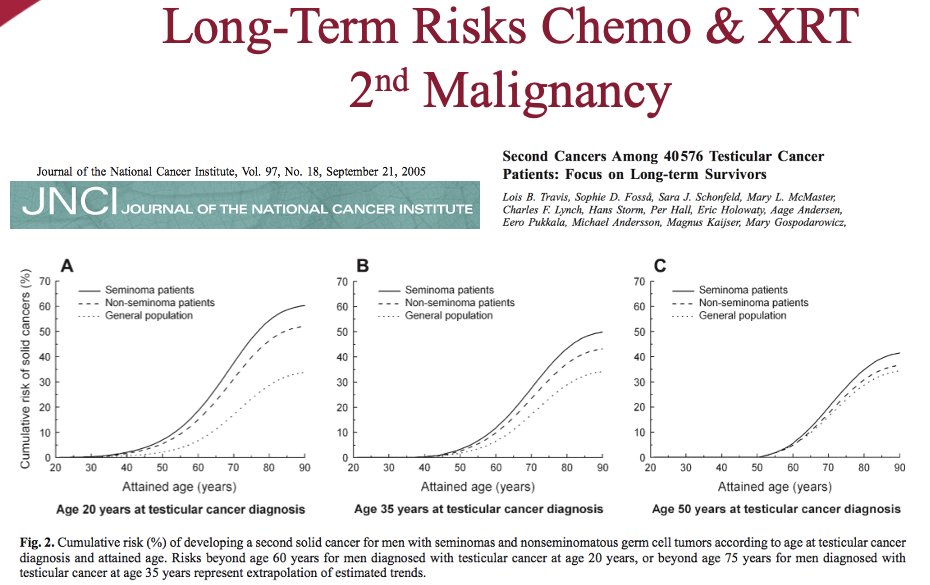
The management of testicular seminoma has long been influenced by historical studies published more than 50 years ago. In 1959, Patton et al. published a series of 510 patients from the Walter Reed Army Medical Center demonstrating that there was a 95% cure rate with orchiectomy and radiotherapy, with radiotherapy administered to the retroperitoneum as well as sites of distant metastases.1 A decade later, Boctor et al demonstrated that survival outcomes were similar irrespective of whether patients, of whom ~40% were stage II-III, underwent an RPLND or radiation to the pelvic and retroperitoneal lymph nodes. However, short-term morbidity was worse with surgery.2 As such, post-orchiectomy radiotherapy became the established standard of care option for such patients.
However, over the past few decades, we have recognized that there are long-term risks for chemotherapy and radiotherapy, namely the risk of secondary malignancy and cardiovascular morbidity. As such, there has been an interest in evaluating alternate treatment approaches for such patients.
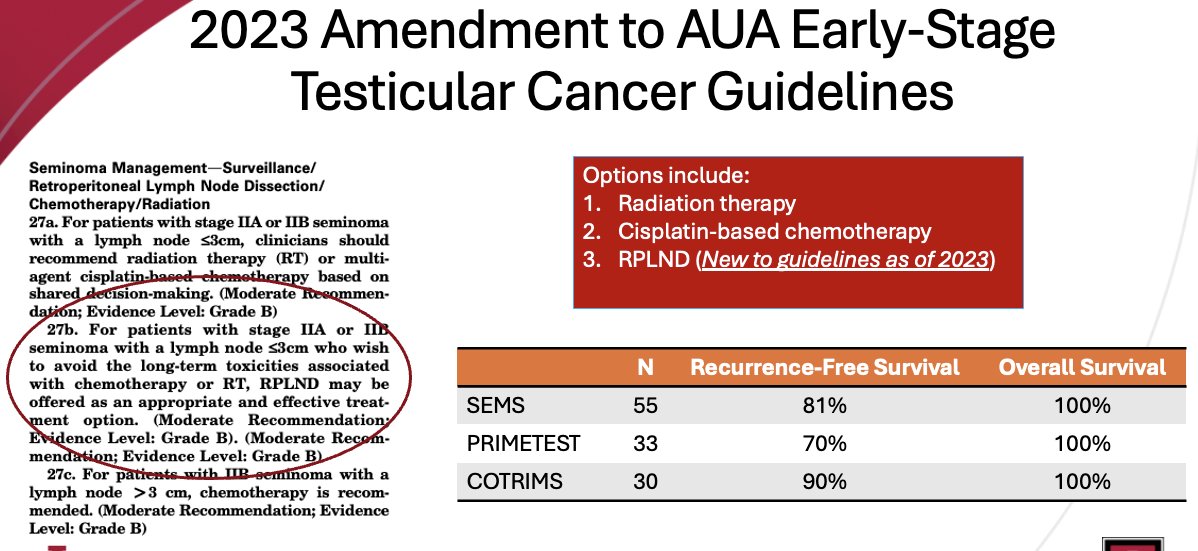
Following the results of three seminal trials in this space (SEMS, PRIMETEST, COTRIMS), the international guidelines have updated their recommendations to acknowledge the role of RPLND as an ‘appropriate and effective treatment option’ for patients with stage IIA or IIB seminoma with lymph nodes ≤3 cm who wish to avoid the long-term toxicities associated with chemotherapy or radiotherapy.
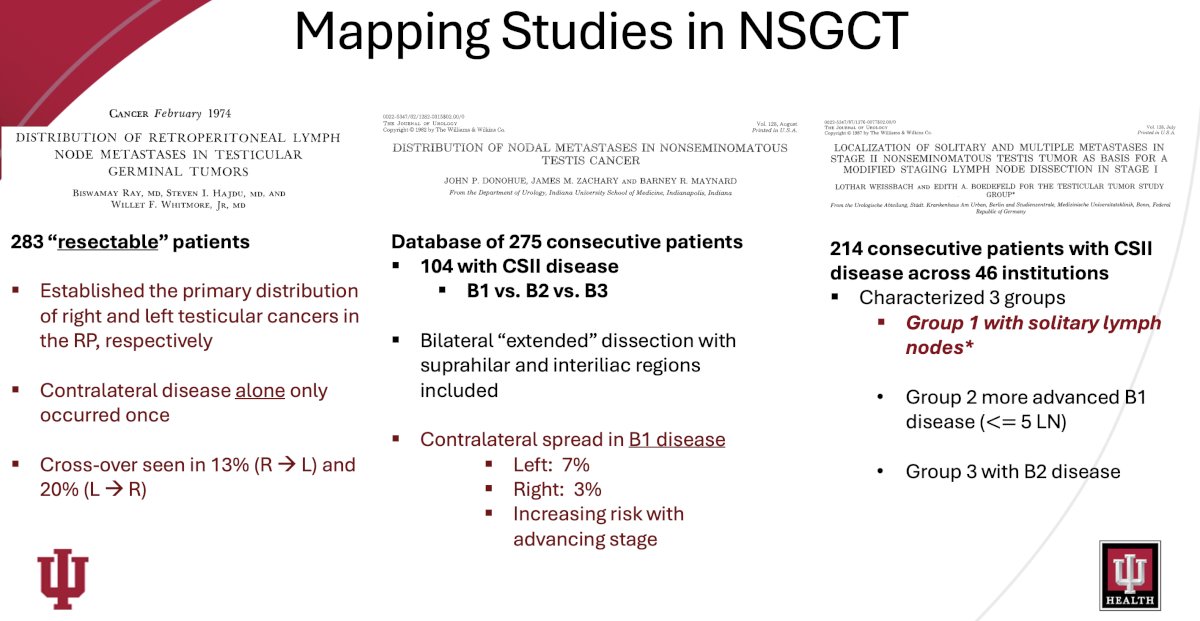
While RPLND has long been established as a treatment options in the management paradigm of non-seminoma patients with stage IIA or IIB disease, are there differences in their patterns of disease spread that make RPLND even more reasonable for similarly stage seminoma patients? What have mapping studies in non-seminomatous germ cell tumors demonstrated? Overall, these studies have:
- Eliminated the suprahilar dissection except in bulky disease
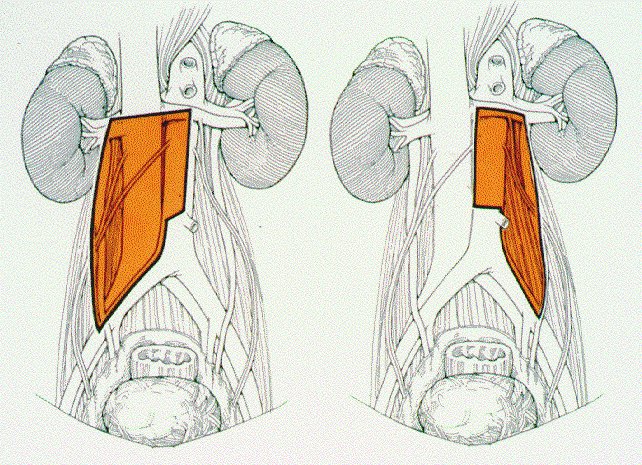
- Established the importance of resection of the ipsilateral gonadal vessels
- Contralateral iliac/interiliac regions are rarely involved
- Defined risk of cross-over to contralateral basins in advancing disease
- Demonstrated the negative impact on fertility with bilateral templates
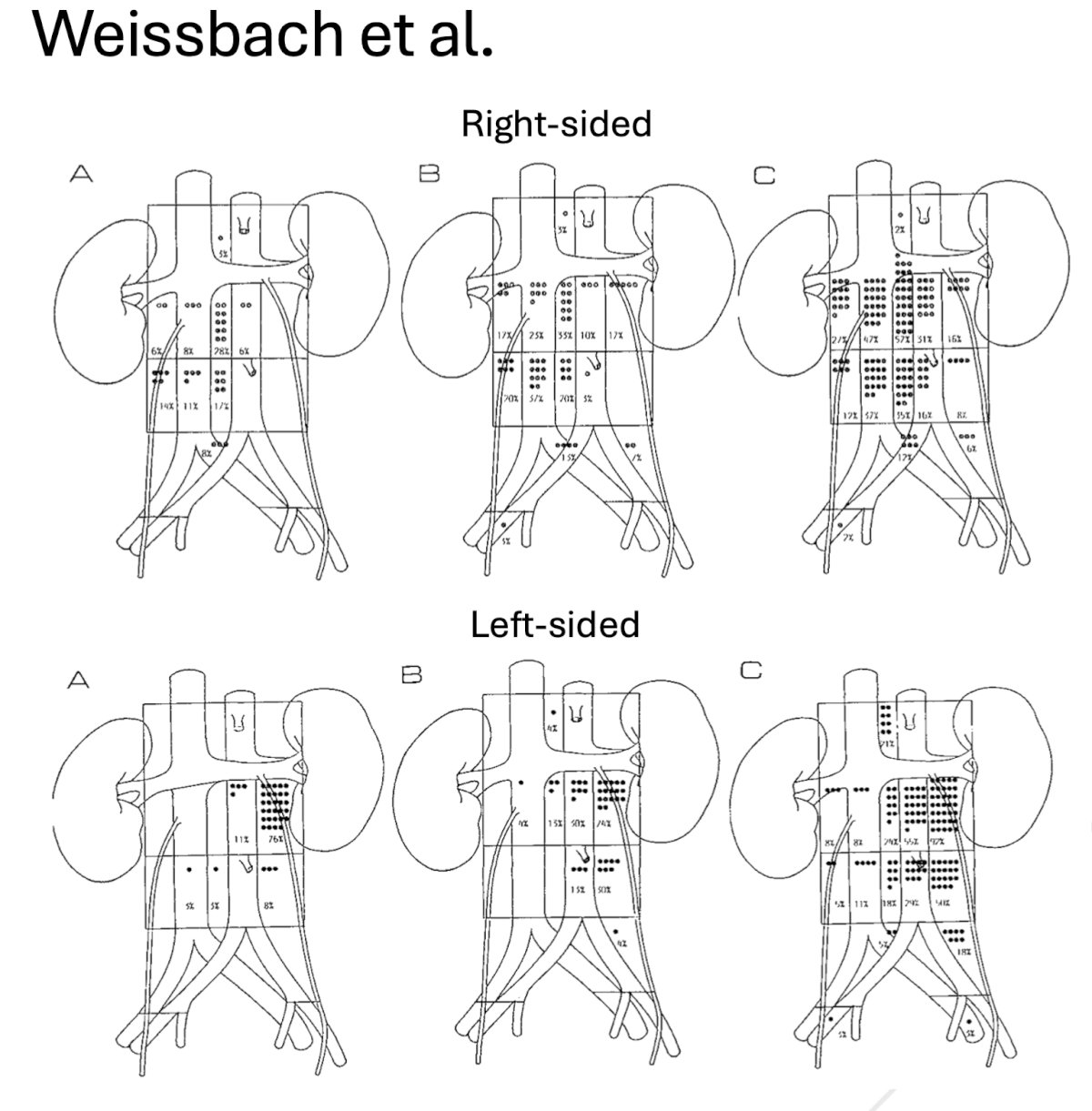
In particular, the mapping study by Weissbach et al. ‘set the stage’ for implementation of modified templates in early-stage disease. In a cohort of 214 patients with stage II disease and metastatic deposits <5 cm, including 74 patients with solitary metastases, Weissbach et al. demonstrated that solitary nodes of the right testis tumor were located with decreasing frequency in the upper and lower interaortocaval, lower paracaval and precaval, upper precaval and right common iliac, upper paracaval and upper preaortic zones. Primary deposits of the left testis tumor were seen predominantly in the upper para-aortic zone. Upper preaortic and lower para-aortic zones were involved infrequently, and other areas were affected only in rare cases. Overall, ipsilateral areas were defined according to primary involvement.3
Importantly, follow-up prospective trials with modified templates have demonstrated that 95–97% of metastatic deposits are confined to the ipsilateral area.
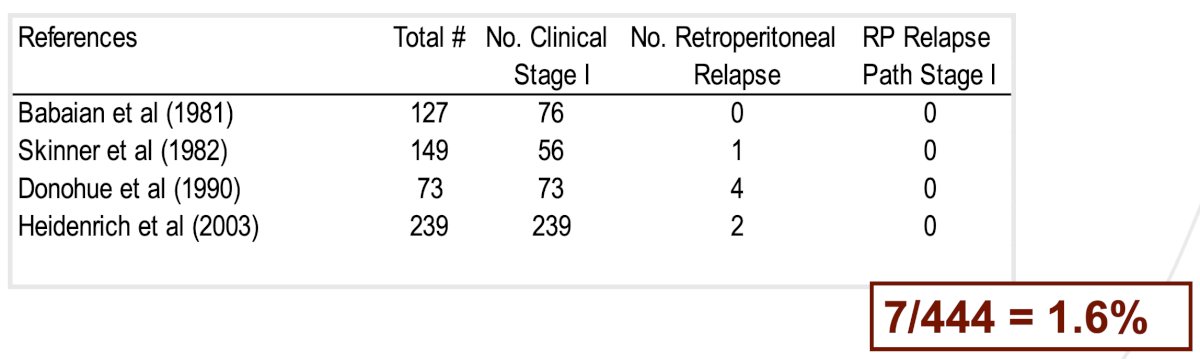
It is important to note that in-field relapses after a primary RPLND are rare, with aggregate estimates of 1.6% reported in the literature.4-7
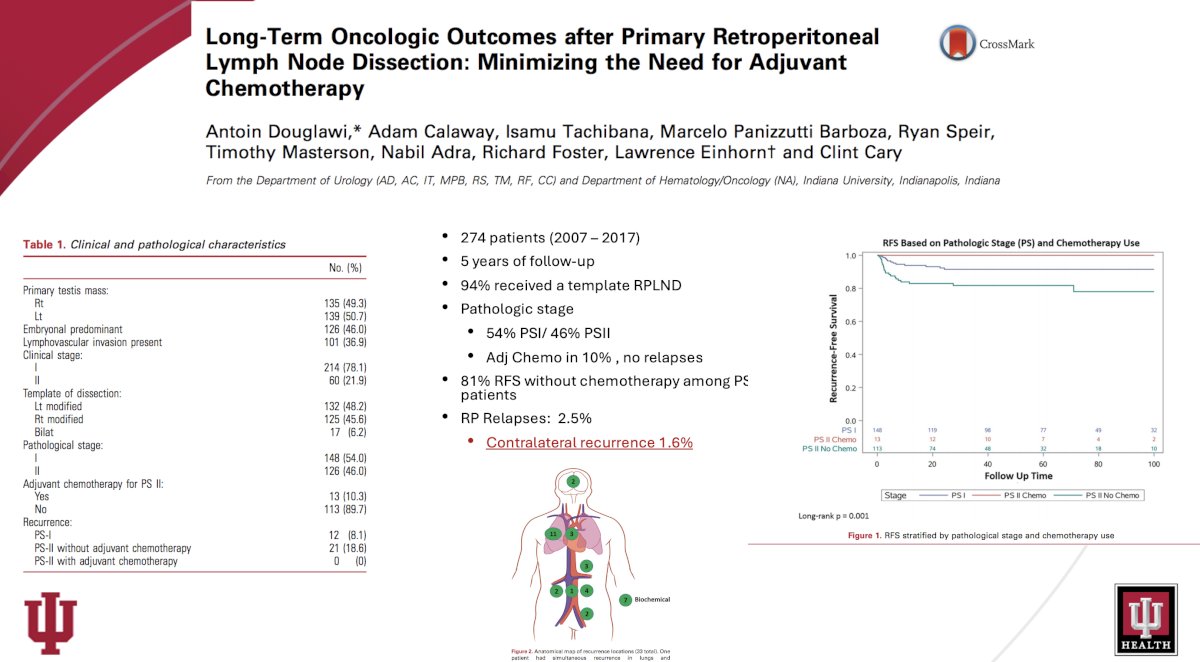
The University of Indiana published its institutional experience with primary RPLND for non-seminomatous germ cell tumors in 2020. They analyzed the oncologic outcomes of men undergoing primary RPLND and characterized the use of adjuvant chemotherapy and template dissections. A total of 274 patients were included in the study. Most men presented with clinical stage I disease (78%). A modified unilateral template was performed in 94%, with the remaining undergoing a bilateral template. Overall, 148 (54%) and 126 (46%) men had pathological stage (PS) I and PS-II disease, respectively. Thirteen patients (10%) with PS-II disease were treated with adjuvant chemotherapy. With a median follow-up of 55 months, only 33 (12%) patients experienced disease recurrence. Of the 113 patients with PS-II disease who did not receive chemotherapy, 21 (19%) had disease relapse and 81% were cured with surgery alone and never had recurrence. No difference in recurrence-free survival was noted between modified and bilateral template dissections, with contralateral recurrences observed in only 1.6%. Based on these results, the study investigators concluded that the use of adjuvant chemotherapy at their center has been minimal during the last decade. The majority (81%) of men with PS-II disease were cured with retroperitoneal lymph node dissection alone and were able to avoid chemotherapy. ‘Modified unilateral template dissection provided excellent oncologic control while minimizing morbidity.8
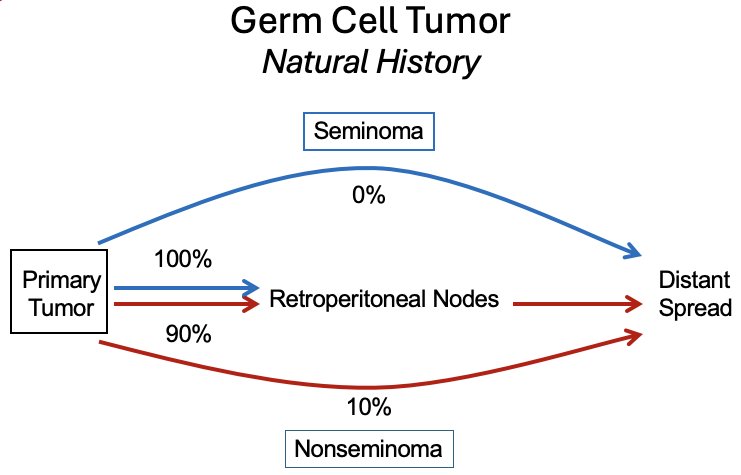
It has been consistently demonstrated that seminomatous tumors follow a stepwise metastatic pattern. While 10% of nonseminomas ‘’bypass’ the retroperitoneum and directly spread to distant metastatic sites, all seminomas demonstrate a stepwise metastatic pattern from the primary site to the retroperitoneal nodes and subsequently distant sites.
What is the contemporary surgical experience in testicular seminoma? As previously noted, there have been three large trials of primary RPLND for stage IIA/B seminomas: SEMS, PRIMETEST, and COTRIMS.
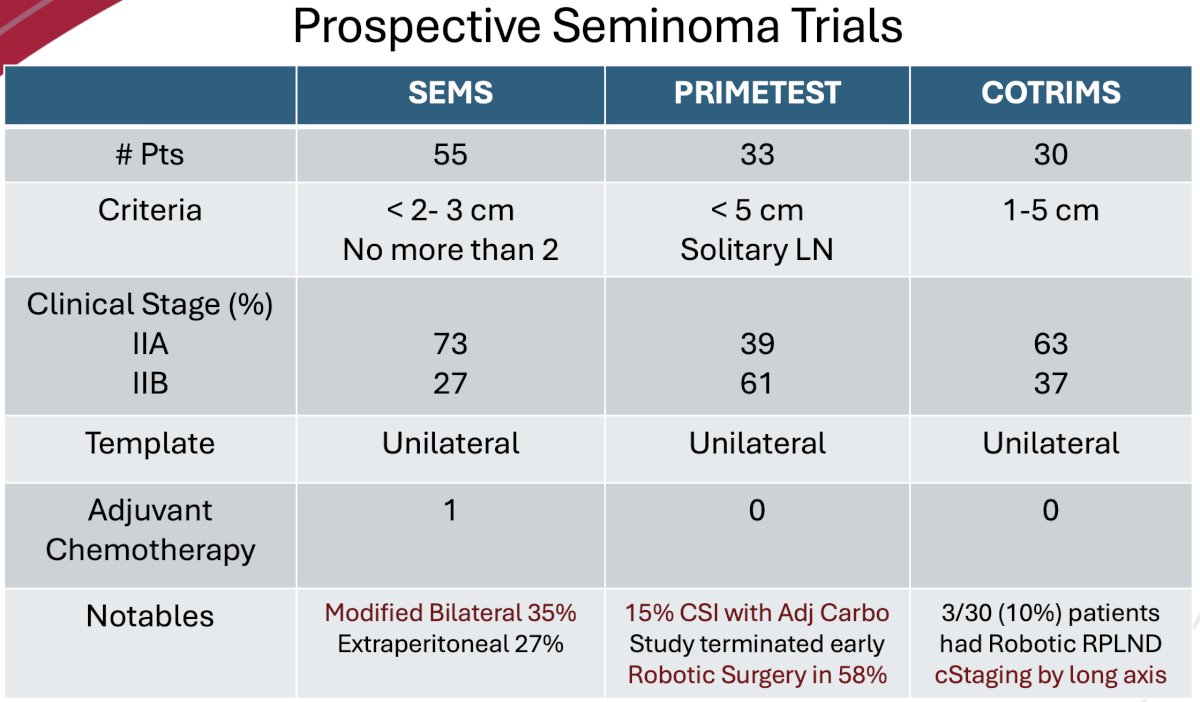
There are some notable differences between the three trials. The inclusion criteria differed:
- SEMS allowed for tumors <3 cm, but no more than 2
- Used the short axis for size limit
- PRIMETEST allowed for <5 cm solitary lymph nodes
- COTRIMS allowed for inclusion of solitary tumor deposits 1-5 cm in the primary landing zone
- For size criteria, this trial used the largest of 3 dimension
SEMS included comparatively more patients with stage IIA disease (73% versus 39–63%). All employed a unilateral template, and only one patient overall received adjuvant chemotherapy (SEMS). Other important differences between the trials included the fact that 35% of patients in the SEMS trial underwent a modified bilateral template. 15% of patients in the PRIMETEST trial had initially presented with CSI disease and had received adjuvant carboplatin – suggesting that these patients may be chemo-resistant. Overall, in terms of surgical approach, 58% of patients in PRIMETEST underwent a robotic RPLND, compared to 10% in COTRIMS and none in SEMS.
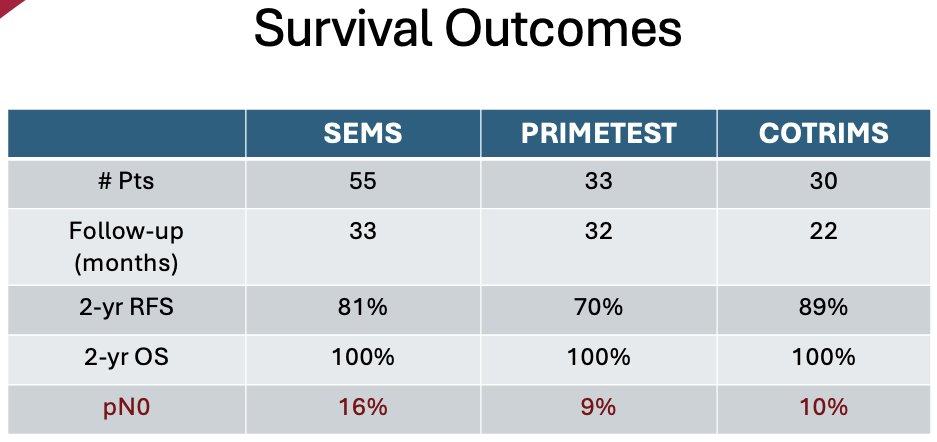
Survival outcomes were similar across the three trials, with patients in the COTRIMS trial having the best 2-year recurrence-free survival rates (89% versus 70% and 81% in PRIMETEST and SEMS, respectively). However, all patients had a 2-year overall survival of 100%. The pN0 rate in these trials ranged from 9% to 16%, suggesting that further work to improve the pre-operative identification of patients that do not have residual viable disease is needed.
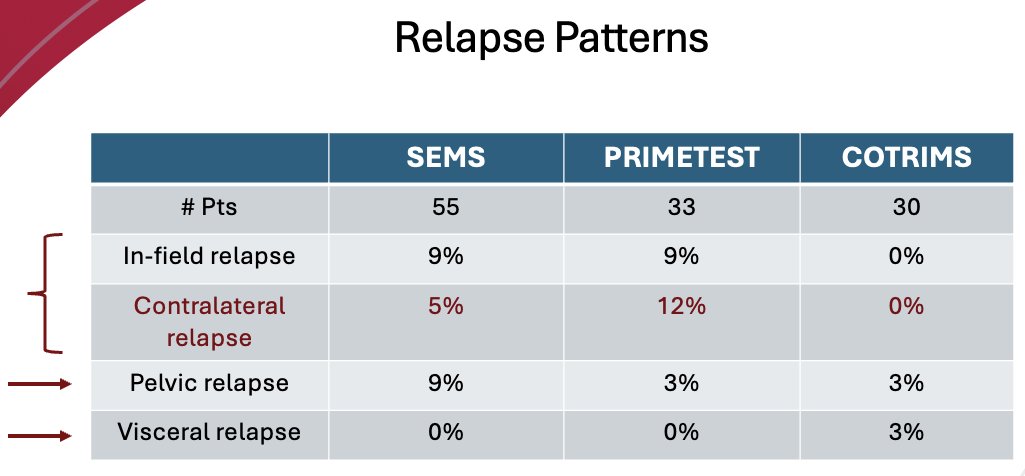
What about relapse patterns? An in-field relapse was observed in 9% of SEMS and PRIMETEST patients, respectively, and a contralateral relapse was observed in 5% and 12% of SEMS and PRIMETEST patients, respectively. Notably, none of the COTRIMS patients experienced an in-field or contralateral relapse. Pelvis relapses were observed in 9% of SEMS patients, but Dr. Masterson argued that this does not suggest that a pelvic lymphadenectomy should be performed in these patients as a result. A visceral relapse was observed in 1 (3%) patient in the COTRIMS trial but in none of the patients from the other two trials.
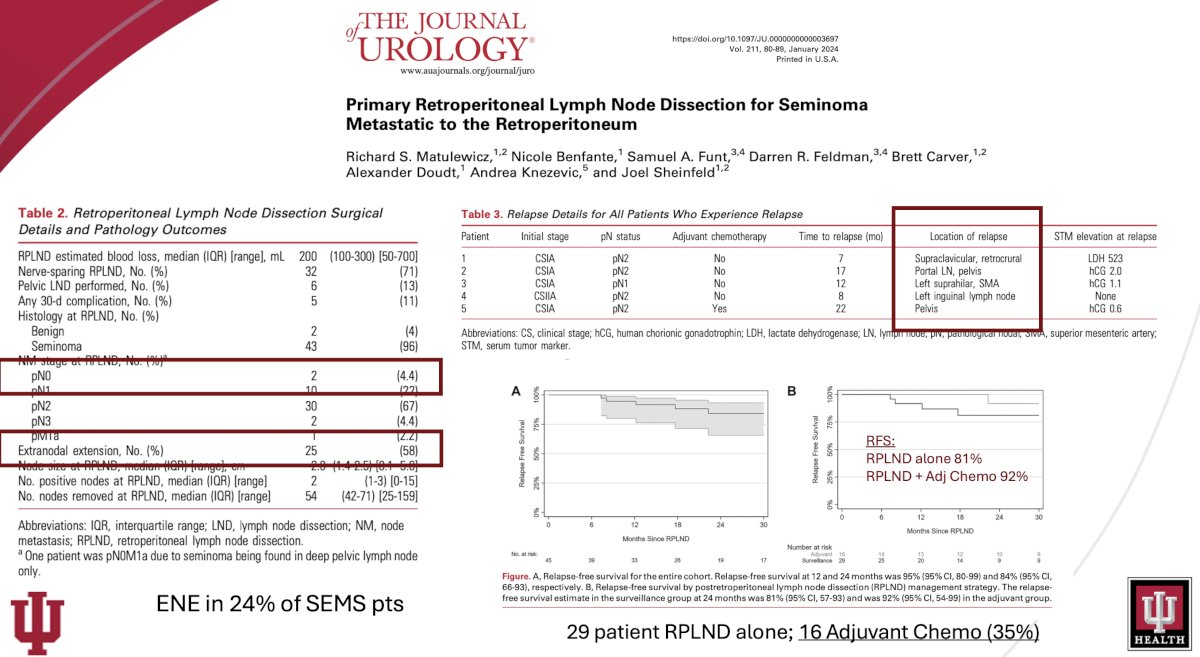
In 2023, the Indiana group published their own single center experience with primary RPLND for stage II seminoma. Patients who underwent primary RPLND for retroperitoneal-limited seminoma from 2014 to 2021 and did not receive adjuvant therapy were included. Of the 67 evaluable patients, 19 were patients from phase II trials. Of these 67 patients, 48, 12, and 7 had CS I, IIA, and IIB disease at the time of diagnosis, respectively. 12/67 underwent upfront RPLND, with the remaining 55/67 managed initially with surveillance, followed by delayed RPLND. A unilateral template was performed in 36/77, and 31/77 underwent a bilateral template.
At a median follow-up of 22.4 months, the 2-year recurrence-free survival for RPLND-only patients without adjuvant chemotherapy was 80.2%. Patients who developed retroperitoneal disease at a period > 12 months had the lowest chance of recurrence, with a 2-year recurrence-free survival of 92.2%. Pathologic nodal stage and high-risk factors such as tumor size > 4 cm or rete testis invasion of the orchiectomy specimen did not affect recurrence. The pN0 rate was 1.3%.12
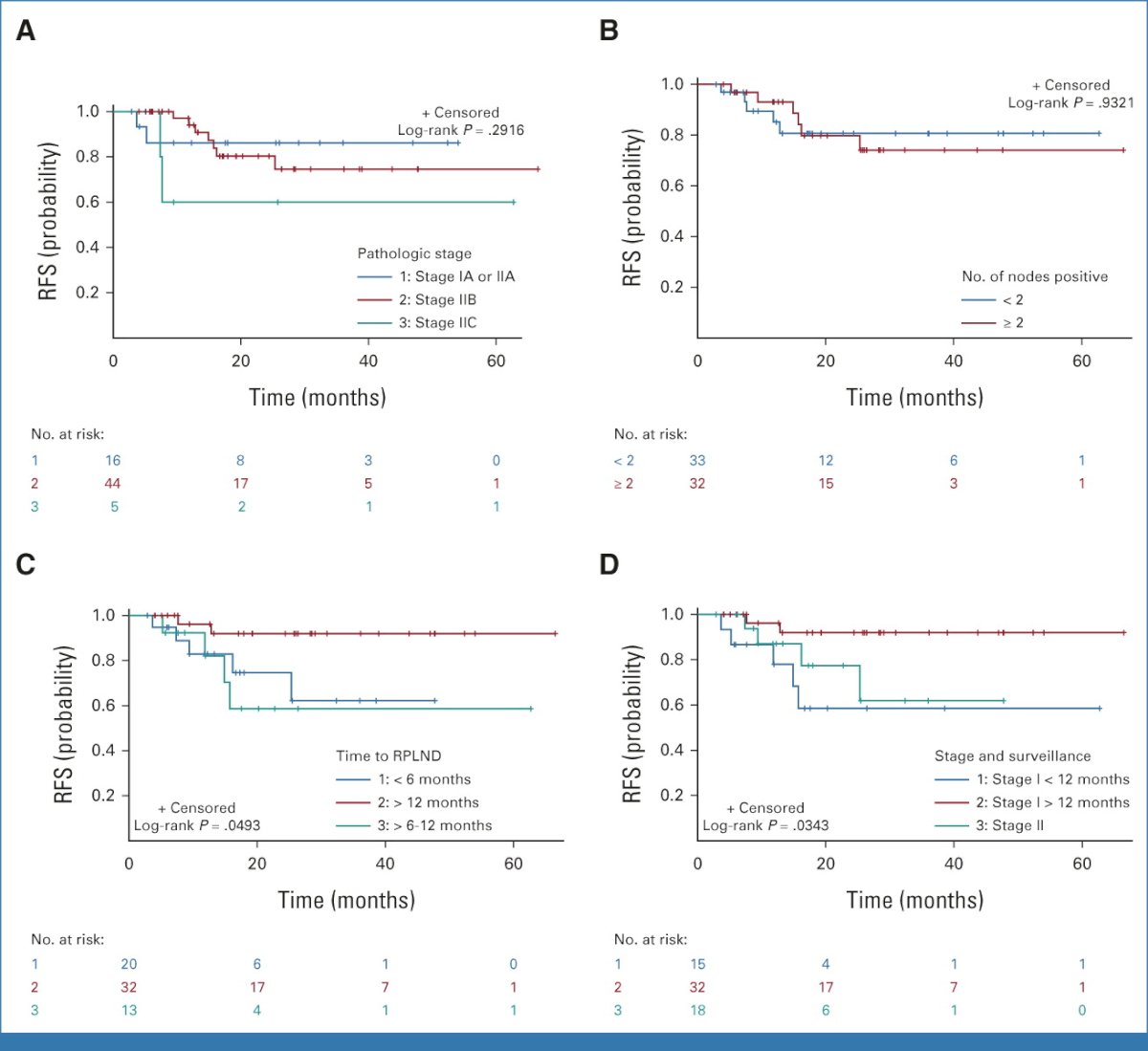
One patient had an infield recurrence, and two patients had contralateral recurrences with one of these two patients having a concurrent pelvic recurrence. Of the 31 patients who had a full bilateral template, only three distant recurrences occurred compared with eight recurrences out of 36 patients undergoing a unilateral template dissection (p=0.17).
Furthermore, for the 31 patients undergoing bilateral template dissection, six patients (19.4%) had contralateral disease on final RPLND pathology, of which four patients had disease noted on preoperative computed tomography scan.12
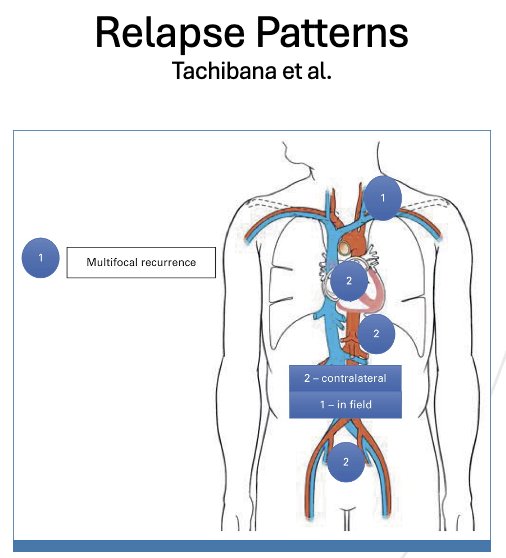
Should we move from a modified unilateral to a bilateral template approach for primary RPLND in patients with metastatic seminoma? Analysis from the University of Indiana cohort suggests that recurrences are significantly higher with a unilateral template (25% versus 15%), both in-field (6% versus 0%) and contralaterally (17% versus 13%).
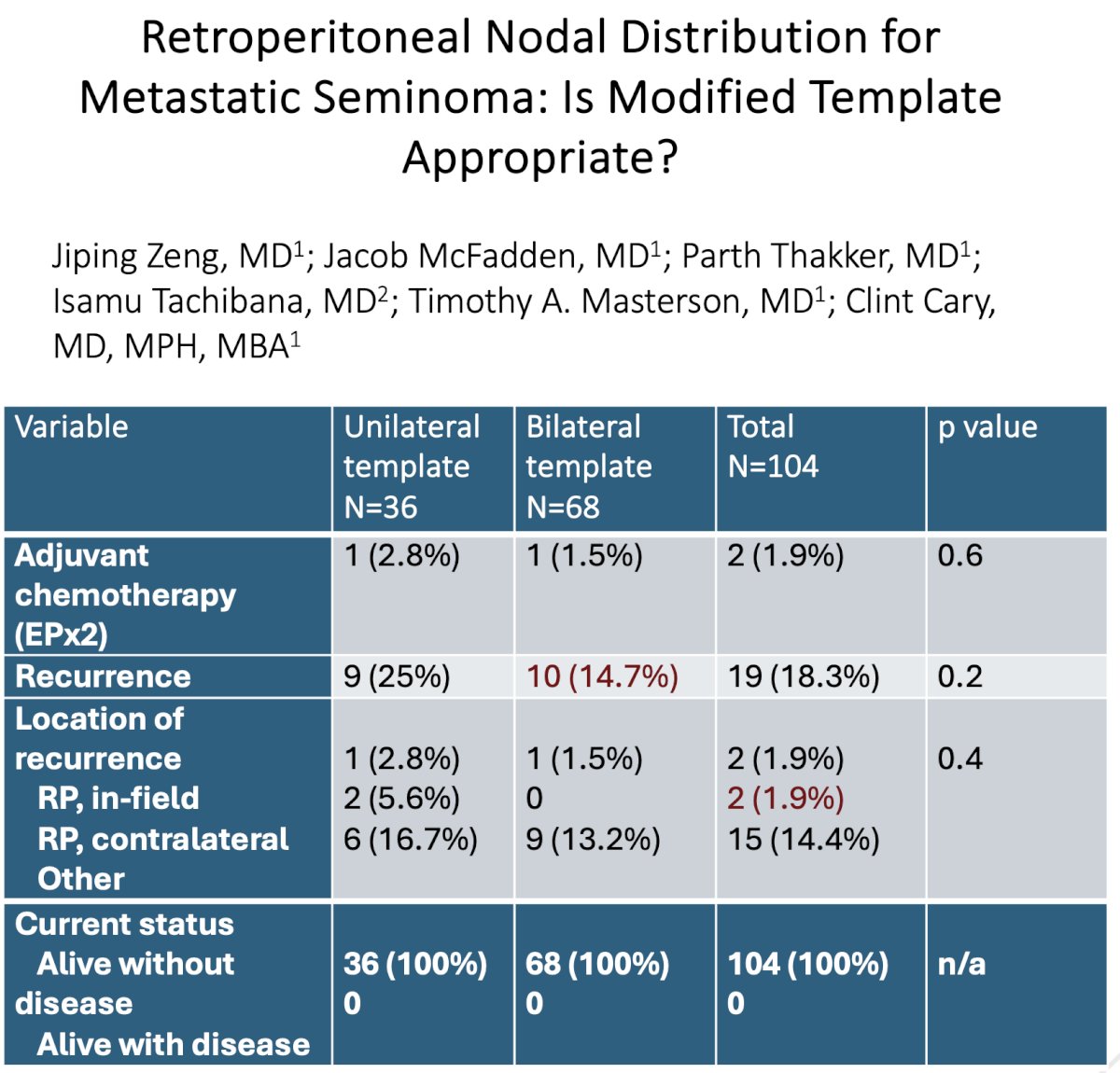
Using data from the MSKCC group, Dr. Masterson noted that one important technical point to consider for minimizing the chances of an in-field recurrence is tissue handling, particularly for patients with extranodal extension (58% in MSKCC experience). This point is particular importance, particularly as robotic RPLNDs become more ubiquitous for the management of such patients.
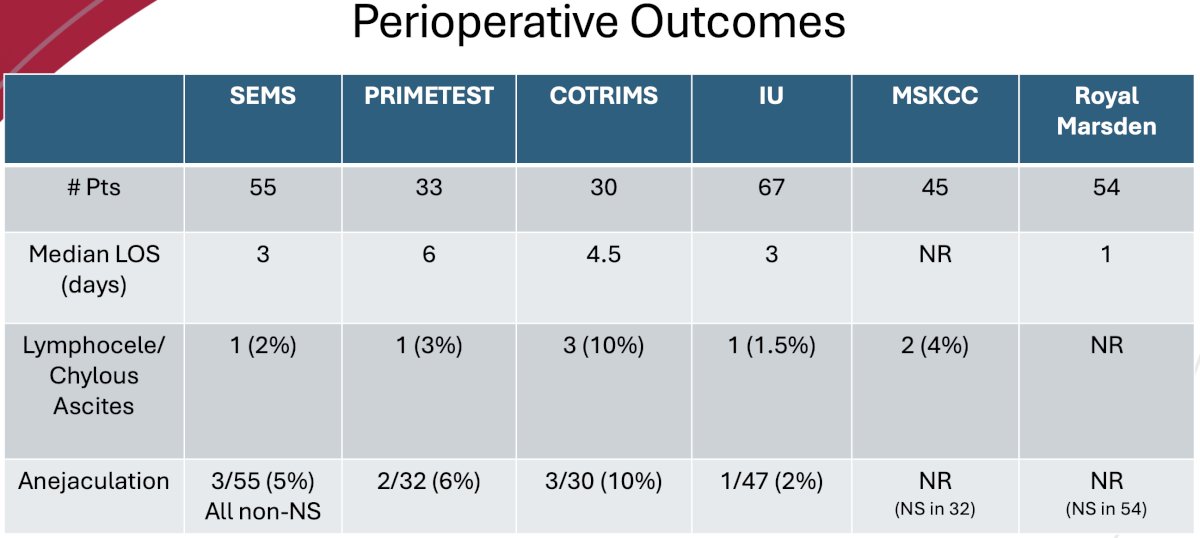
Overall, it appears that the perioperative outcomes for CS IIA/B seminoma patients who undergo a primary RPLND are favorable, with median lengths of stay of ≤3 days, rare occurrences of lymphoceles/chylous ascites, and mostly preserved ejaculatory function.
Dr. Masterson concluded his presentation with the following take home messages:
- The recurrence-free survival with primary RPLND for stage IIA/B seminomas is 80-90%
- pN0 rates appear to be decreasing over time and can be further reduced with:
- Judicious use of repeat imaging/initial surveillance in equivocal pts
- Biomarkers (miRNA 371, ctDNA, etc.)
- Notable observations include:
- Higher rates of contralateral disease/crossover (13-16%)
- Greater risk of pelvic/inter-iliac disease/relapse (~4-10%)
- There appear to be improved survival outcomes with:
- Full left or bilateral templates
- Time to relapse >12 months
- Earlier stage disease
Presented by: Timothy Masterson, MD, Professor, Department of Urology, Indiana University, Indianapolis, IN
Written by: Rashid K. Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, between the 3rd and 6th of December, 2024.
References:
- Patton JF, Hewitt CB, Malis N. Diagnosis and Treatment of Tumors of the Testis. JAMA. 1959; 171(16):2194-8.
- Boctor ZN, Kurohara SS, Badib AO, Murphy GP. Current Results from Therapy of Testicular Tumors. Cancer. 1959; 24(5):870-5.
- Weissbach L, Boedefeld EA. Localization of solitary and multiple metastases in stage II nonseminomatous testis tumor as basis for a modified staging lymph node dissection in stage I. J Urol. 1987; 138(1):77-82.
- Babaian RJ, Johnson DE, Llamas L, Ayala AG. The significance of microvascular invasion in testicular cancer. J Urol. 1981;17:126.
- Skinner DG, Lieskovsky G, Pritchett TR, Boyd SD, Skinner EC. The role of lymphadenectomy in the management of bladder cancer. J Urol. 1982;127:1107.
- Donohue JP, Rowland RG, Bihrle R, et al. Retroperitoneal lymph node dissection in testicular cancer. J Urol. 1990;144:287.
- Heidenreich A, Albers P, Hartmann M, Kliesch S. Complications of primary retroperitoneal lymphadenectomy in non-seminomatous testicular cancer. J Urol. 2003;169:1710.
- Douglawi A, Calaway A, Tachibana I, et al. Long-Term Oncologic Outcomes after Primary Retroperitoneal Lymph Node Dissection: Minimizing the Need for Adjuvant Chemotherapy. J Urol. 2020; 204(1):96-103.
- Daneshmand S, Cary C, Masterson T, et al. Surgery in Early Metastatic Seminoma: A Phase II Trial of Retroperitoneal Lymph Node Dissection for Testicular Seminoma With Limited Retroperitoneal Lymphadenopathy. J Clin Oncol. 2023; 41(16):3009-18.
- Hiester A, Che Y, Lusch A, et al. Phase 2 Single-arm Trial of Primary Retroperitoneal Lymph Node Dissection in Patients with Seminomatous Testicular Germ Cell Tumors with Clinical Stage IIA/B (PRIMETEST). Eur Urol. 2023; 84(1):25-31.
- Heidenreich A, Paffenholz P, Hartmann F, et al. Retroperitoneal Lymph Node Dissection in Clinical Stage IIA/B Metastatic Seminoma: Results of the COlogne Trial of Retroperitoneal Lymphadenectomy In Metastatic Seminoma (COTRIMS). Eur Urol Oncol. 2024; 7(1):122-7.
- Tachibana I, Alabd A, Tong Y, et al. Primary Retroperitoneal Lymph Node Dissection for Stage II Seminoma: Is Surgery the New Path Forward? J Clin Oncol. 2023; 41(23):3930-8.


