(UroToday.com) The 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, between December 3 and December 6, 2024, was host to the Testis Cancer Session. Dr. Meredith Metcalf discussed the Top Papers in Testicular Cancer in 2024.
Dr. Metcalf began by highlighting that testicular cancer therapy is one of the greatest success stories in oncology, with a 95% cure rate. This success is a result of significant advancements since the 1950s-1960s when radiation was first introduced. The addition of cisplatin, vinblastine, and bleomycin in 1977 improved the cure rate to 70%, and cisplatin was FDA-approved for testicular cancer in 1978. By 1980, tumor markers (AFP, HCG, LDH) became integral for diagnosis and follow-up. The introduction of BEP chemotherapy and active surveillance in 1987 marked another milestone. Despite numerous advances in other GU cancers, testicular cancer treatment has seen fewer paradigm shifts due to its high cure rate.
In 2024, three key areas of research in testicular cancer are worth of highlighting:
- Prognostication in Clinical Stage I (CS I) Seminoma.
- Treatment Paradigms in Early Metastatic Seminoma (Primary RPLND):
- MicroRNA (miRNA)
The recurrence rates in CS I seminoma range from 4-30% and clinical practice guidelines highlight as risk factors tumors> 4cm and rete testis invasion.
Dr. Metcalf highlighted two key papers reporting prognostic factors for relapse in patients with CS I seminoma. One is based on the prospective Danish Testicular Cancer Database,1 and the other comes from the EAU Guidelines Panel's analysis of patient data to identify prognostic risk groups for CS I seminoma.2
The EAU Guidelines Panel analyzed data from 1,016 CS I seminoma patients undergoing surveillance across nine institutions. The study examined tumor size, RTI, age, pre-orchiectomy marker levels, lymphovascular invasion (LVI), and tumor focality as prognostic factors. While tumor size >4 cm and RTI have been used in prior trials for risk-adapted management, they are poor predictors when used alone, leading to overtreatment in approximately 70% of patients. Cut points were selected to maximize the identification of low and high-risk groups, based on relapse probabilities of <10% and >25%, respectively.
After a median follow-up of 7.7 years, 149 (14.7%) patients had relapsed. The categorization of tumor size (2 cm, >2-5 cm, >5 cm), the presence of RTI, and LVI led to the formation of three risk groups: low (56.4%), intermediate (41.3%), and high (2.3%) risk. This model outperformed the one based on tumor size and RTI alone, with a Harrell's C index of 0.65 vs. 0.61 as illustrated in the table below: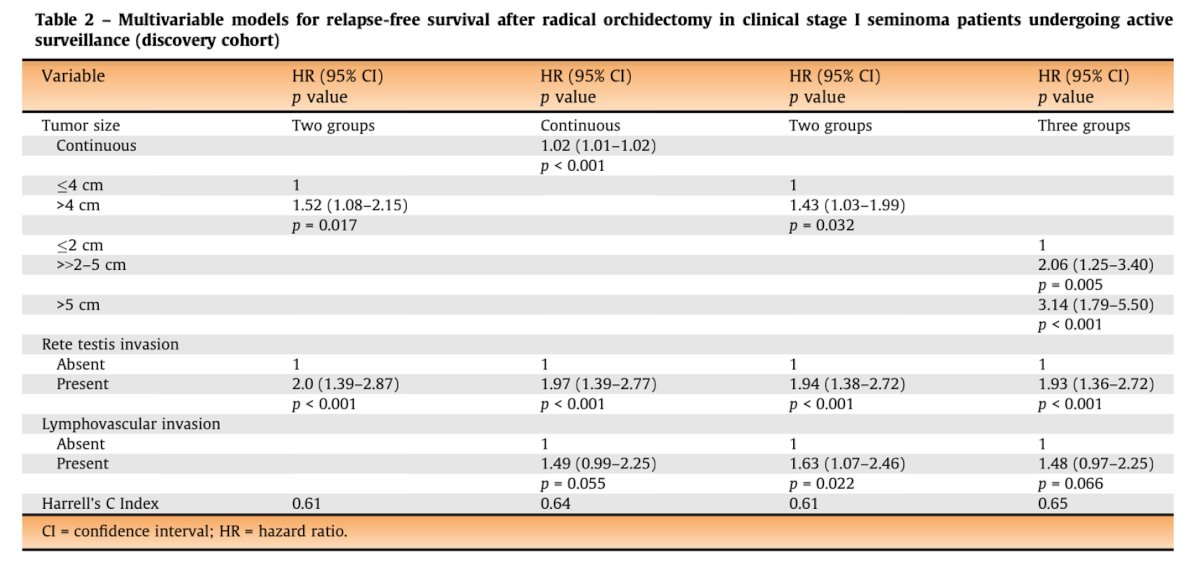
The 5-year cumulative probability of relapse was 8%, 20%, and 44%, for low, intermediate and high-risk groups, respectively.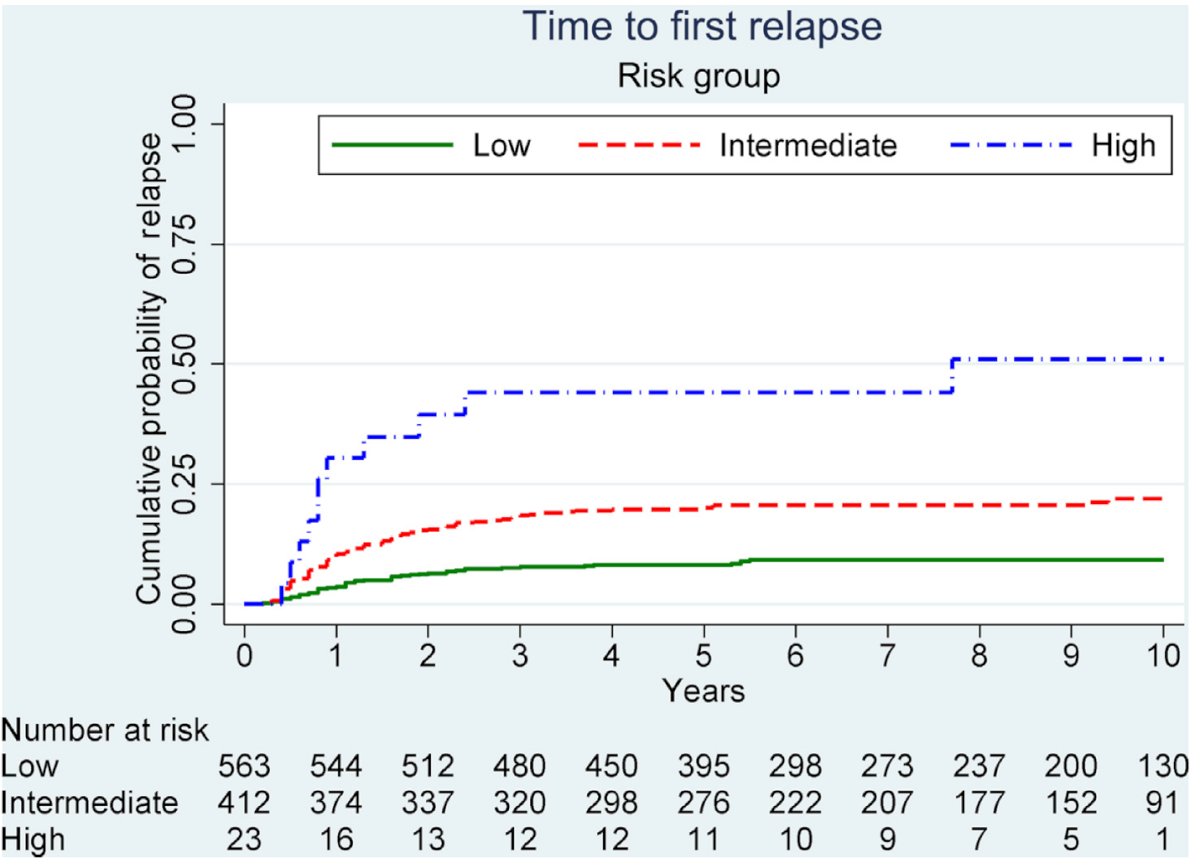
The low- and intermediate-risk groups identified in the EAU study were successfully validated in an independent cohort of 285 patients from the Swiss Austrian German Testicular Cancer Cohort Study, further supporting the model's prognostic value.2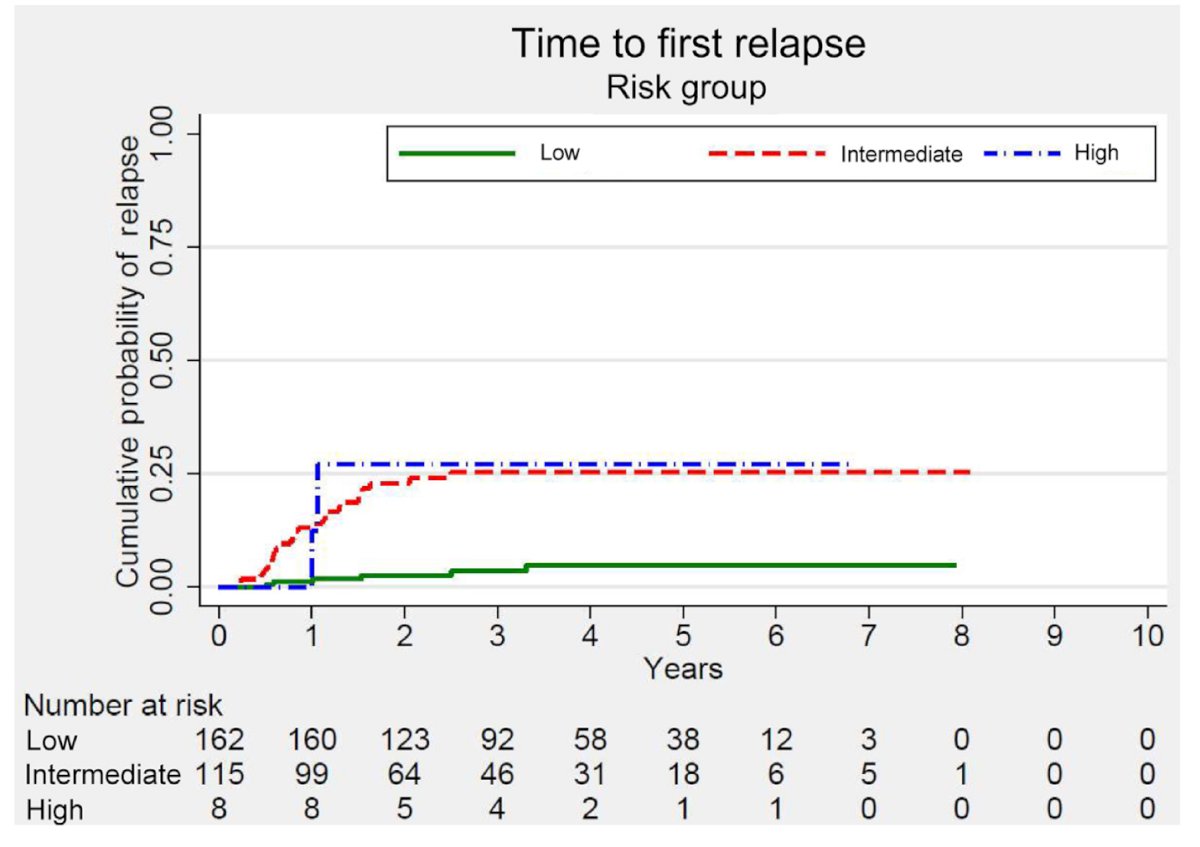
Dr. Metcalf mentioned that while the new prognostic model for CS I seminoma may not change the treatment approach since it still suggests overtreatment in 40-55% of cases, it can improve patient counseling by better defining relapse risks. This allows for more informed discussions and could lead to risk-adapted regimens for surveillance or treatment intensification.
For early metastic seminoma, radiation or chemotherapy has been the standard of care for decades, effectively curing most patients. However, these treatments carry long-term risks, such as secondary cancers, cardiovascular issues, metabolic syndrome, and infertility, particularly concerning for young patients with decades of potential life ahead.
Recently, there has been a renewed interest in surgery in this setting as primary retroperitoneal lymph node dissection (RPLND), has regained interest as an alternative to reduce long-term toxicity and avoid the cumulative effects of radiation and chemotherapy.
Dr. Metcalf discussed the COTRIMS study, a prospective, single-arm Phase 2 trial led by Axel Heidenreich in Cologne, which included patients with clinical stage IIA/B seminoma. Notably, the study measured retroperitoneal lymph node dimensions three-dimensionally, using the largest axis for assessment. In cases with equivocal findings, patients were re-scanned 6-8 weeks later. A modified unilateral template was used for nerve-sparing retroperitoneal lymph node dissection. A total of 30 patients underwent surgery, with 27 undergoing open RPLND and three robot-assisted procedures.3
Among the 25 patients who underwent RPLND, 83% had metastatic seminoma, 6.7% had embryonal carcinoma, and 10% had benign pathology as shown in the table below: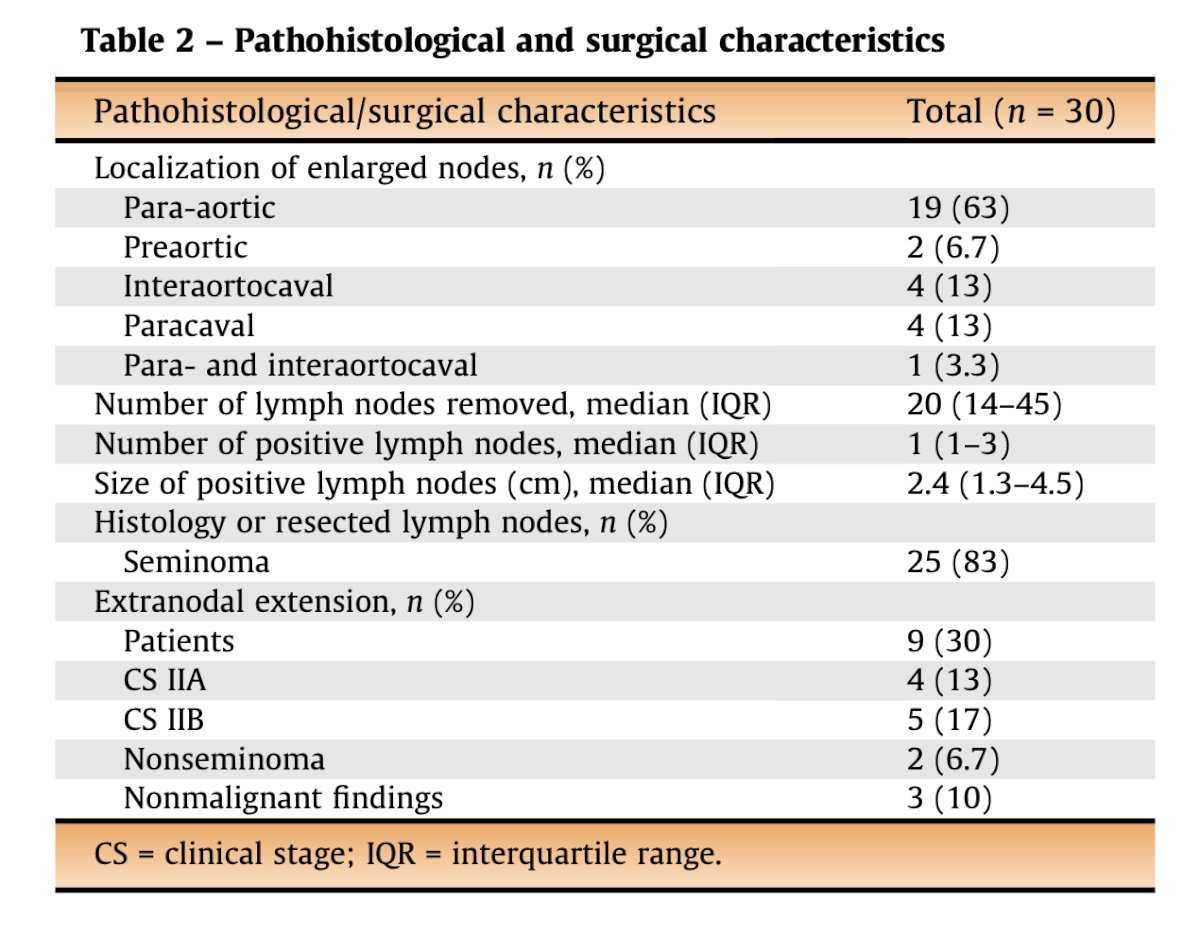
Three patients (10%) experienced an outfield relapse at a median of 6.5 months and were successfully salvaged with systemic chemotherapy. Notably, 90% of patients required no further therapy, and no high-grade complications were reported.3 An important takeaway from this study is the need for a better understanding of recurrence patterns to guide salvage treatments following pRPLND for clinical stage II seminoma. Further research is needed to improve patient management and tailor interventions based on recurrence risk.
In the CS I setting, key clinical dilemmas remain, such as accurately detecting relapses, identifying them earlier than with conventional methods, and determining whether elevated postoperative miRNA levels can serve as predictors of relapse.
Dr. Metcalf presented a multicenter study involving 258 patients, of whom 189 had seminoma and 69 had non-seminoma with CS I disease. These patients were prospectively followed with surveillance for a median of 18 months. During this time, serial measurements of serum miRNA371 levels, in addition to serum tumor markers and imaging, were used to monitor disease progression.
In this study, miRNA371 was able to detect earlier relapses in 28% of patients, but it did not result in a significant reduction in the time to diagnosis. The graphic below shows the highest miRNA371 measurements for each patient at corresponding follow-up time points. Red dots represent relapsing patients, and green dots represent non-relapsing patients. Interestingly, eight patients showed elevated miRNA371 levels without experiencing a relapse.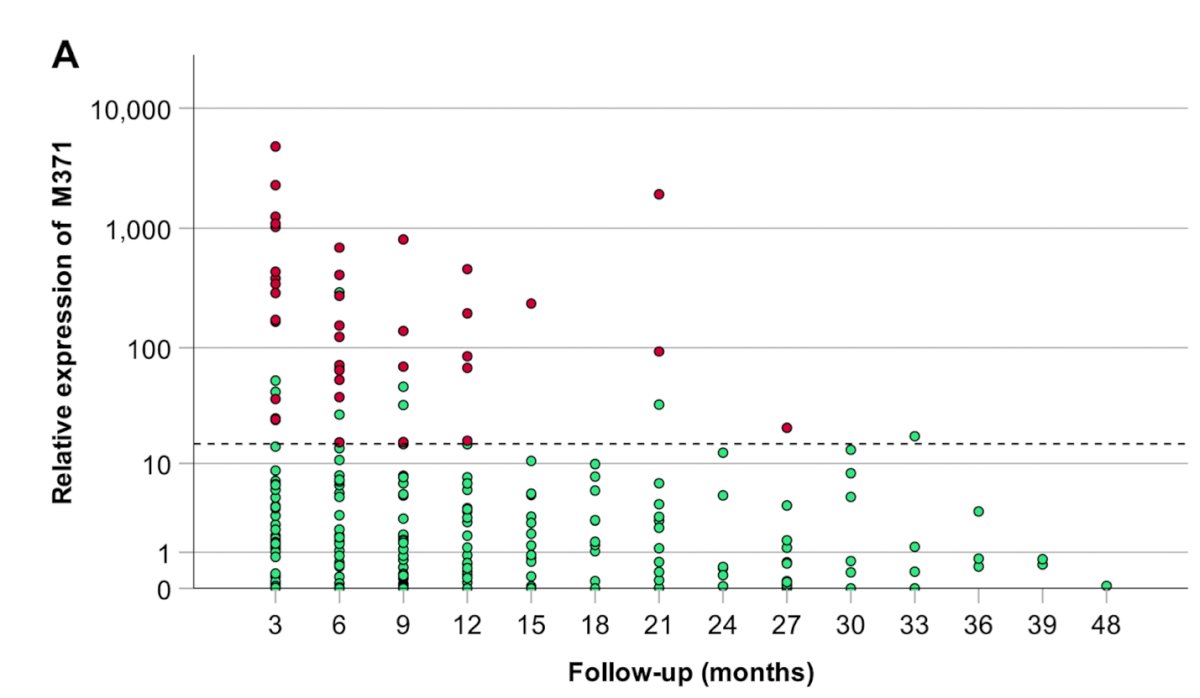
Postoperative miRNA371 levels were not predictive of future relapse in CS I TGCTs.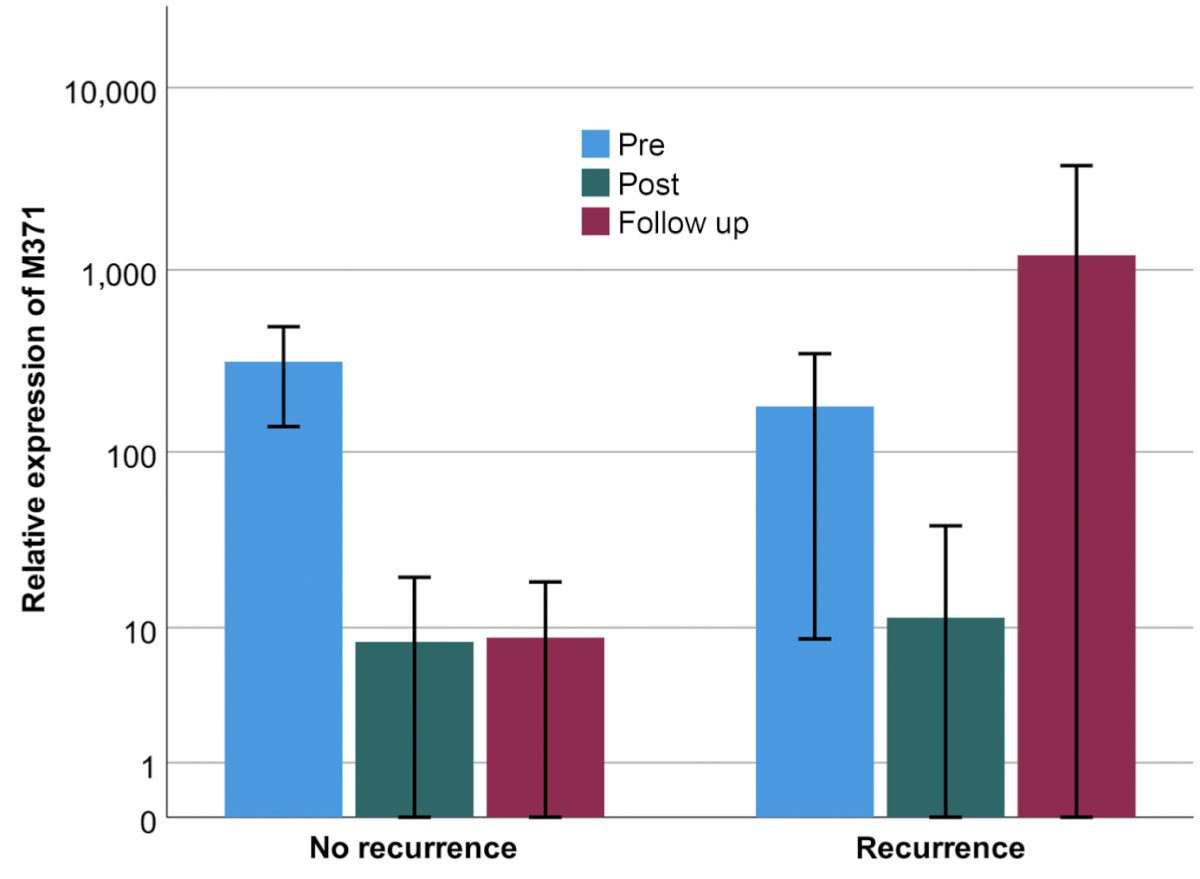
Limitations of this study include:
- Relapses were easily detectable by imaging
- There is no histologic data available on recurrences in this study.
- The optimal reference quantity (RQ) for miRNA371 is still being determined.
- The performance of miRNA371 may vary depending on factors such as population demographics, histology, and disease stage.
Dr. Metcalf reviewed a study evaluating miRNA371 for predicting final pathology in 24 patients undergoing primary nerve-sparing RPLND for CS IIA/B TGCTs. Histology confirmed metastatic TGCT in 22 out of 24 patients (91.7%), and positive miRNA371 findings were seen in 20 of these 22 patients (90.9%). Among the four patients with negative miRNA371 results, one had no malignancy, one had lymphoma, one had pure teratoma (as expected), and one had a microscopic seminoma. The sensitivity and specificity for the overall population as 95.7% and 100%, respectively.5 This highlights the potential for miRNA for predicting the presence of low volume retroperitoneal lymph node metastases in marker-negative TGCT.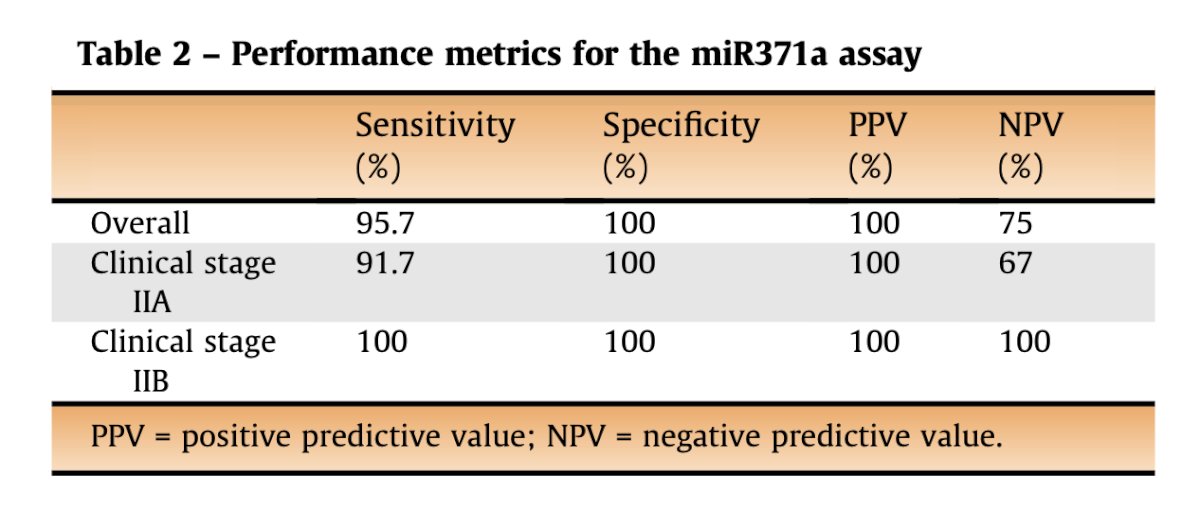
Lastly, a study led by Dr. Dieckmann was presented, this was a multicenter, prospective diagnostic study involving 180 GCT patients who underwent post-chemotherapy resection of residual masses, irrespective of their location. Among the patients, 15 had seminoma and 165 had non-seminoma. Pathological findings revealed that 33% of the resected masses were necrosis or fibrosis, 42% were teratoma, and 25% showed viable cancer.
The sensitivity of miRNA371 in predicting the presence of viable disease in residual masses after chemotherapy was found to be 68.9%, with a specificity of 99.3%, and an area under the curve (AUC) of 0.813.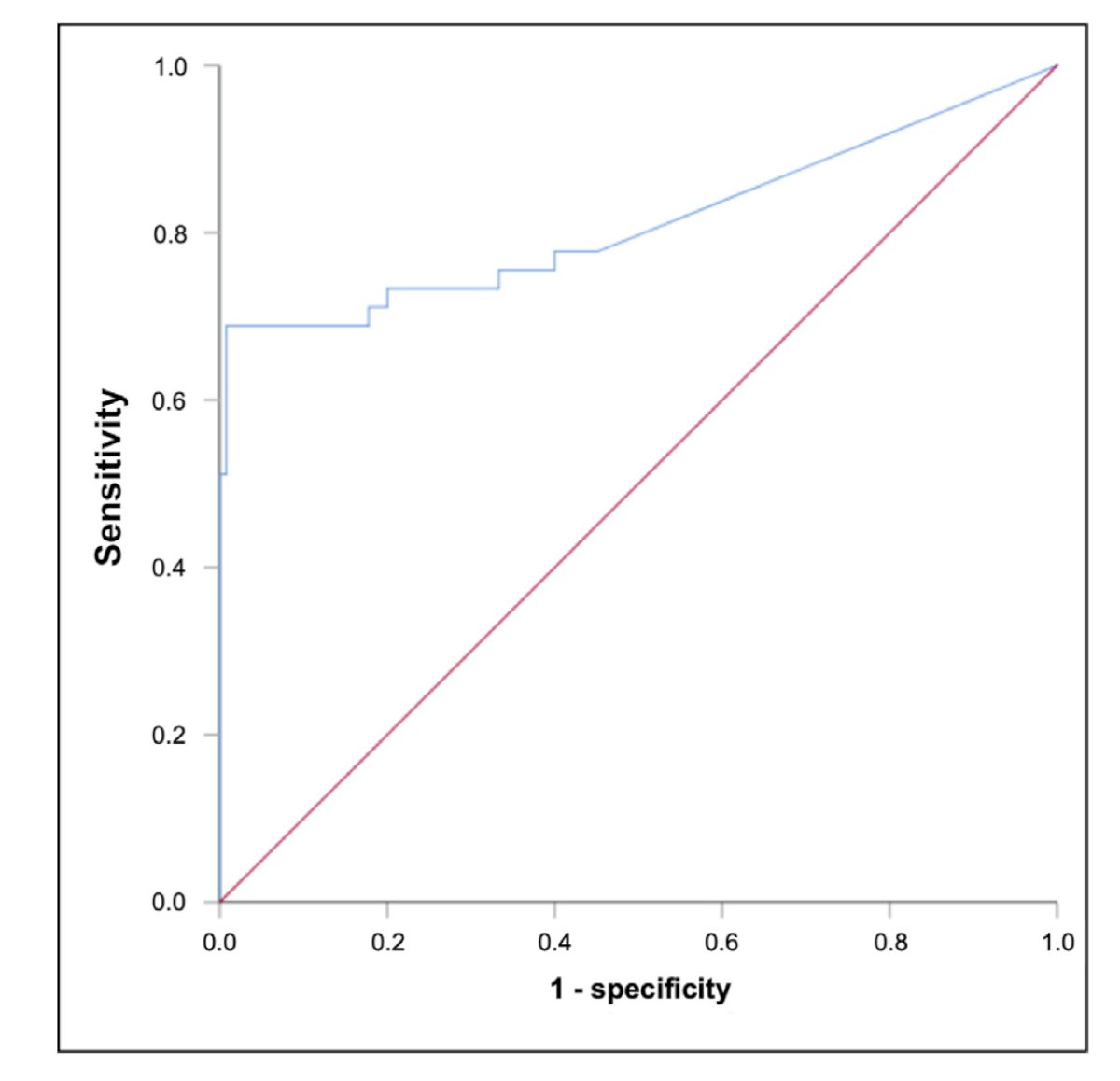
Notably, sensitivity varied based on the percentage of viable cancer in the mass. For specimens with more than 50% viable disease, 85.7% showed elevated miRNA371 levels, whereas only 33.3% of specimens with 10% viable cancer had elevated levels.6 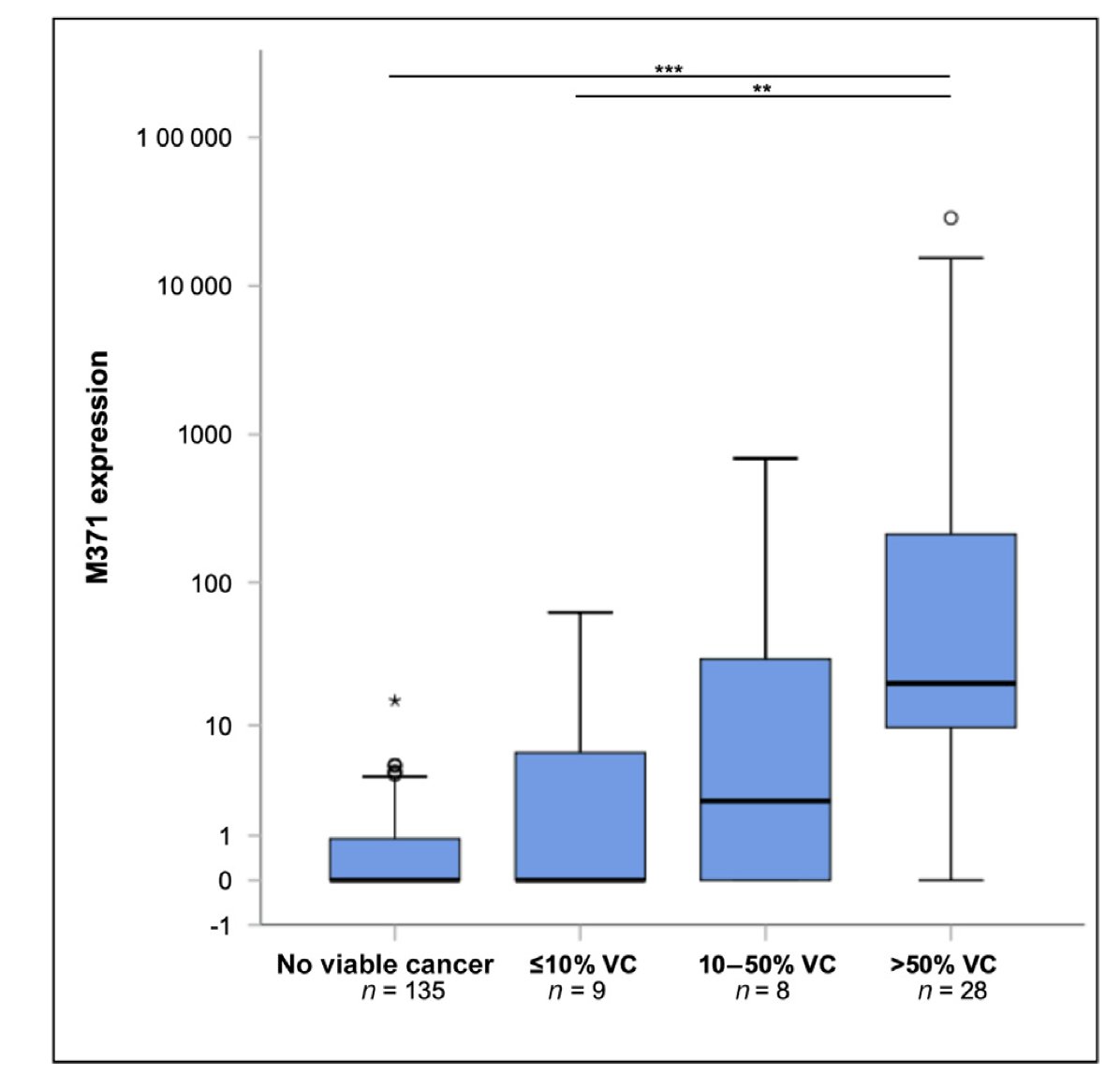
Presented by: Meredith Metcalf, MD, Assistant Professor of Surgery, Washington University School of Medicine in St. Louis. St. Louis, MO.
Written by: Julian Chavarriaga, MD – Urologic Oncologist at Cancer Treatment and Research Center (CTIC) Luis Carlos Sarmiento Angulo Foundation via Society of Urologic Oncology (SUO) Fellow at The University of Toronto. @chavarriagaj on Twitter during the 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, between the 3rd and 6th of December, 2024.
References:- Wagner T, Toft BG, Lauritsen J, Bandak M, Christensen IJ, Engvad B, Kreiberg M, Agerbæk M, Dysager L, Rosenvilde JJ, Berney D, Daugaard G. Prognostic Factors for Relapse in Patients With Clinical Stage I Testicular Seminoma: A Nationwide, Population-Based Cohort Study. J Clin Oncol. 2024 Jan 1;42(1):81-89. doi: 10.1200/JCO.23.00959. Epub 2023 Sep 8. PMID: 37683134.
- Boormans JL, Sylvester R, Anson-Cartwright L, Glicksman RM, Hamilton RJ, Hahn E, Daugaard G, Lauritsen J, Wagner T, Avuzzi B, Nicolai N, Del Muro XG, Aparicio J, Stalder O, Rothermundt C, Fischer S, Laguna MP. Prognostic Factor Risk Groups for Clinical Stage I Seminoma: An Individual Patient Data Analysis by the European Association of Urology Testicular Cancer Guidelines Panel and Guidelines Office. Eur Urol Oncol. 2024 Jun;7(3):537-543. doi: 10.1016/j.euo.2023.10.014. Epub 2023 Nov 10. PMID: 37951820.
- Belge G, Dumlupinar C, Nestler T, Klemke M, Törzsök P, Trenti E, Pichler R, Loidl W, Che Y, Hiester A, Matthies C, Pichler M, Paffenholz P, Kluth L, Wenzel M, Sommer J, Heinzelbecker J, Schriefer P, Winter A, Zengerling F, Kramer MW, Lengert M, Frey J, Heidenreich A, Wülfing C, Radtke A, Dieckmann KP. Detection of Recurrence through microRNA-371a-3p Serum Levels in a Follow-up of Stage I Testicular Germ Cell Tumors in the DRKS-00019223 Study. Clin Cancer Res. 2024 Jan 17;30(2):404-412. doi: 10.1158/1078-0432.CCR-23-0730. PMID: 37967143; PMCID: PMC10792362.
- Heidenreich A, Paffenholz P, Hartmann F, Seelemeyer F, Pfister D. Retroperitoneal Lymph Node Dissection in Clinical Stage IIA/B Metastatic Seminoma: Results of the COlogne Trial of Retroperitoneal Lymphadenectomy In Metastatic Seminoma (COTRIMS). Eur Urol Oncol. 2024 Feb;7(1):122-127. doi: 10.1016/j.euo.2023.06.004. Epub 2023 Jul 10. PMID: 37438222.
- Seelemeyer F, Pfister D, Pappesch R, Merkelbach-Bruse S, Paffenholz P, Heidenreich A. Evaluation of a miRNA-371a-3p Assay for Predicting Final Histopathology in Patients Undergoing Primary Nerve-sparing Retroperitoneal Lymphadenectomy for Stage IIA/B Seminoma or Nonseminoma. Eur Urol Oncol. 2024 Jun;7(3):319-322. doi: 10.1016/j.euo.2023.10.021. Epub 2023 Nov 4. PMID: 37932157.
- Dieckmann KP, Grobelny F, Soave A, Che Y, Nestler T, Matthies C, Heinzelbecker J, Winter A, Heidenreich A, Niemzok T, Dumlupinar C, Angerer M, Wülfing C, Paffenholz P, Belge G. Serum Levels of MicroRNA-371a-3p for Predicting the Histology of Postchemotherapy Residual Masses of Germ Cell Tumours. Eur Urol Focus. 2024 Sep;10(5):851-857. doi: 10.1016/j.euf.2024.05.002. Epub 2024 May 9. PMID: 38729824.


