(UroToday.com) The 2024 SUO annual meeting included a urothelial carcinoma session, featuring a presentation by Dr. Brian Hu discussing long-term outcomes of primary chemoablation of low-grade upper tract urothelial carcinoma with UGN-101. Endoscopically guided ablation is commonly used to treat low grade upper tract urothelial carcinoma. While effective, ablation is not typically durable, requiring long-term endoscopic surveillance associated with potential complications.
Topical adjuvant aqueous chemotherapy has a modest effect on disease recurrence following local ablation, likely due to drug dilution and rapid evacuation secondary to physiologic urine flow. Topical gel therapy has, therefore, been developed, with the intention of sustained drug delivery. In the phase 3 OLYMPUS trial,1 UGN-101, a reverse thermal gel containing mitomycin (4 mg/mL) used as primary treatment for low grade upper tract urothelial carcinoma, resulted in clinically significant disease eradication. At the 2024 SUO annual meeting, Dr. Hu and colleagues reported long term outcomes of patients who achieved a complete response in OLYMPUS, defined as a negative 3 month ureteroscopic evaluation, negative cytology, and negative for cause biopsy.
Patients who participated in the OLYMPUS trial and achieved a complete response after 6 weekly doses of UGN-101 were followed up for 12 months after initial complete response. Those with complete response at study completion were eligible for long-term follow-up in BL007. Outcomes of interest in BL007 included duration of response and number of patients with disease recurrence or progression. There was no protocol-specified intervention or treatment, and there were no protocol-specified visits or evaluations. Supervising physicians provided semiannual updates on patients’ disease status for up to 4 years, including disease recurrence, progression, or death:
Of the 71 patients enrolled in OLYMPUS (68% male, 87% White, median age 71 years), 42 achieved complete response 4–6 weeks after UGN-101 last dose, approximately 3 months after study start. Among the 41 patients followed after initial complete response (1 withdrew consent), median follow-up was 28.1 months (95% CI 13.1–57.5). Twenty patients (49%) had long-term follow-up in BL007 (median 53.3 months [95% CI 27.9–65.3]), with baseline characteristics as follows: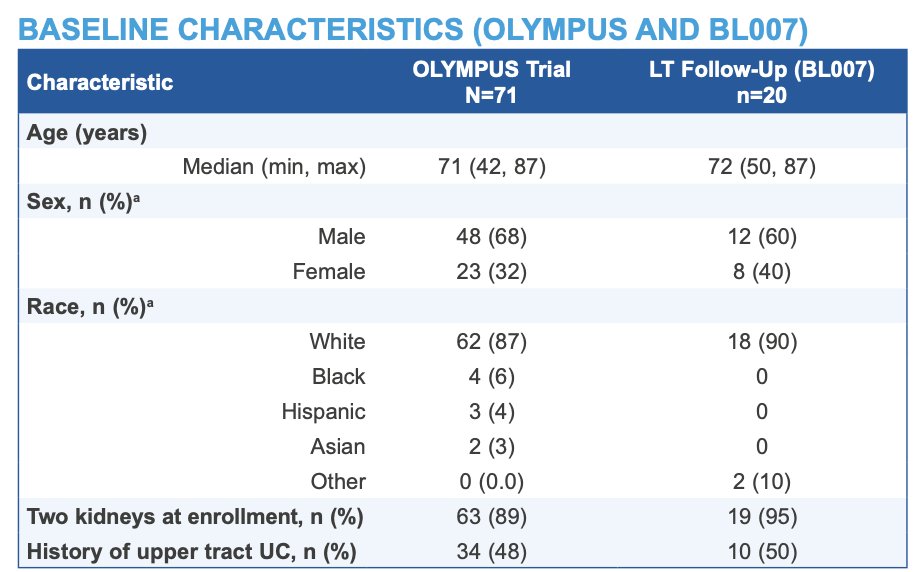
The Swimmer plot for duration of response in OLYMPUS and BL007 is as follows: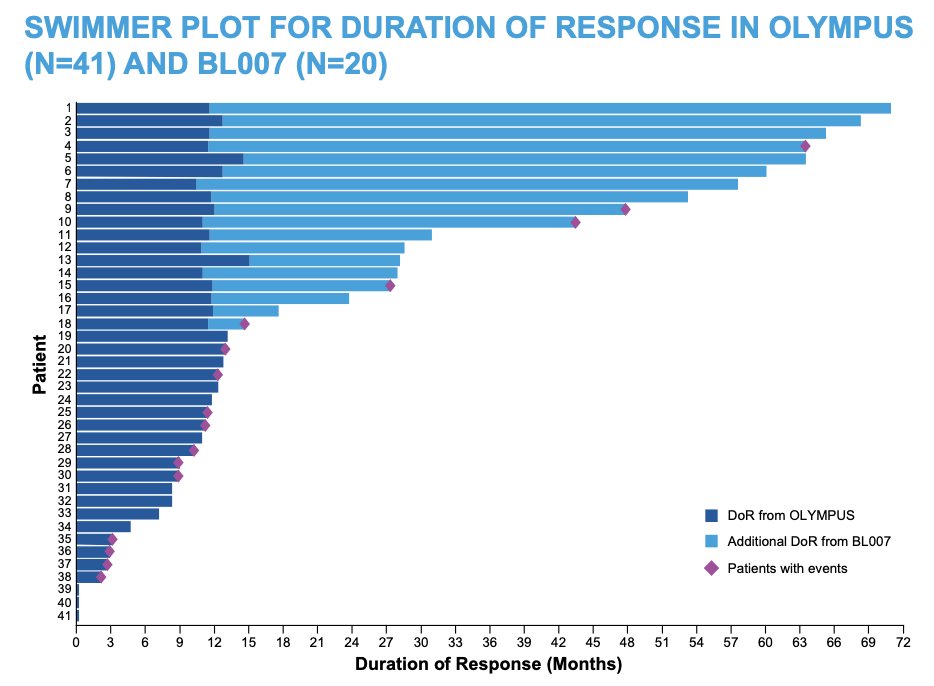
Among the 20 patients in BL007, 75% had no evidence of recurrence at the last follow-up, with median duration of response not estimable (95% CI 43.5–not estimable) due to a low event rate: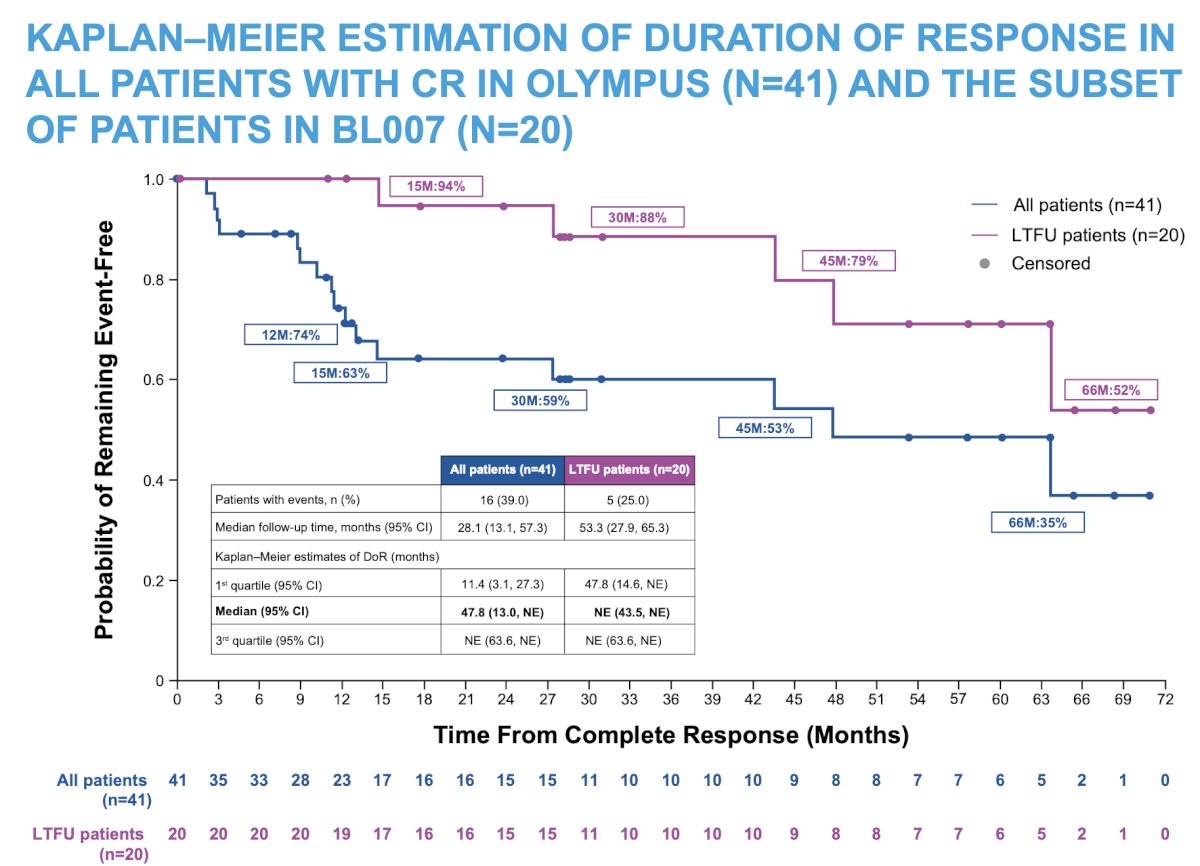
Of the 41 patients with complete response in OLYMPUS, 16 (39%) had documented events: 10 (24.4%) had urothelial carcinoma tumor recurrence, and 6 (14.6%) patients died (not treatment-related). Of the patients evaluated in the long-term follow-up BL007 study, two (10%) experienced urothelial carcinoma tumor recurrence and 3 (15%) died, 2 unknown reasons and 1 secondary to septic shock from E. coli bacteremia and acute hypoxemic respiratory failure; no deaths were related to study treatment. There were no reported progressions to high-grade disease: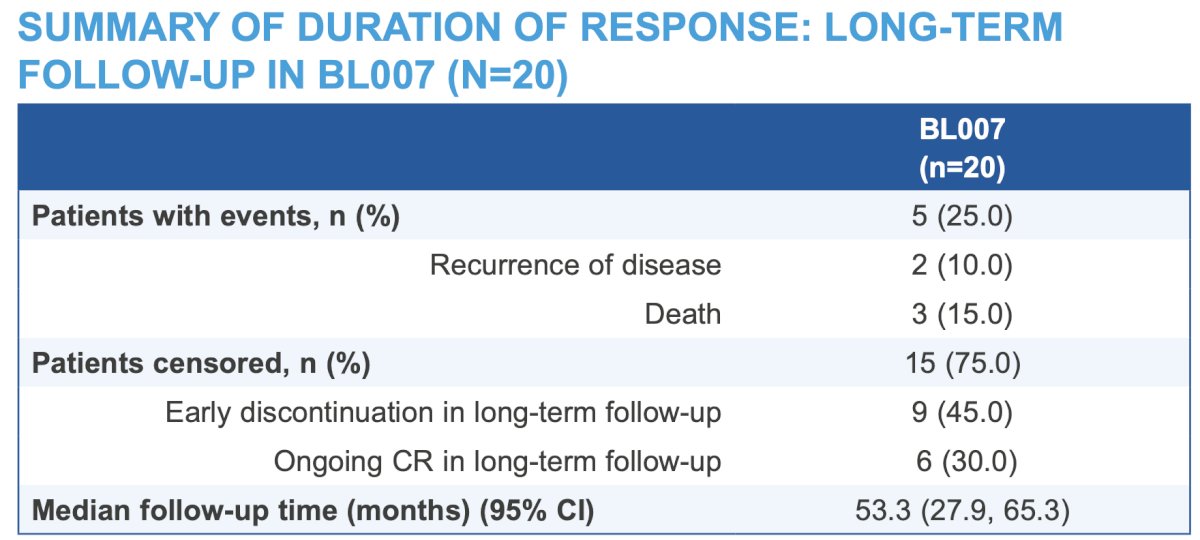
Dr. Hu concluded his presentation discussing long-term outcomes of primary chemoablation of low-grade upper tract urothelial carcinoma with UGN-101 with the following take home messages:
- Patients with low grade upper tract urothelial carcinoma who achieved complete response after receiving primary chemoablation therapy with UGN-101 in the OLYMPUS trial experienced excellent long-term response, with a median duration of response of almost 4 years
- In the subset of patients entering the BL007 long-term follow-up study, the median duration of response was not estimable due to the low event rate
- This favorable data on durability augments a growing body of literature supporting the use of UGN-101 as primary treatment for low grade upper tract urothelial carcinoma
Presented by: Brian Hu, MD, Loma Linda University, Loma Linda, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Society of Urologic Oncology (SUO) Annual Meeting, Dallas, TX, Tues, Dec 3 – Fri, Dec 6, 2024.
References:


