(UroToday.com) The 2024 SUO annual meeting included a urothelial carcinoma session, featuring a presentation by Dr. Vitaly Margulis discussing the ENLIGHTED phase 3 study assessing the efficacy and safety of padeliporfin vascular targeted photodynamic therapy (VTP) for the treatment of low-grade upper tract urothelial carcinoma. Padeliporfin VTP is a combination product:
- The drug: padeliporfin (a photosensitizer), is intravenously administered
- A device: a laser light delivery system, emits near-infrared (NIR) light at 753 nm, and an optic fiber delivers the light to the target lesion(s) in the upper tract urothelium:
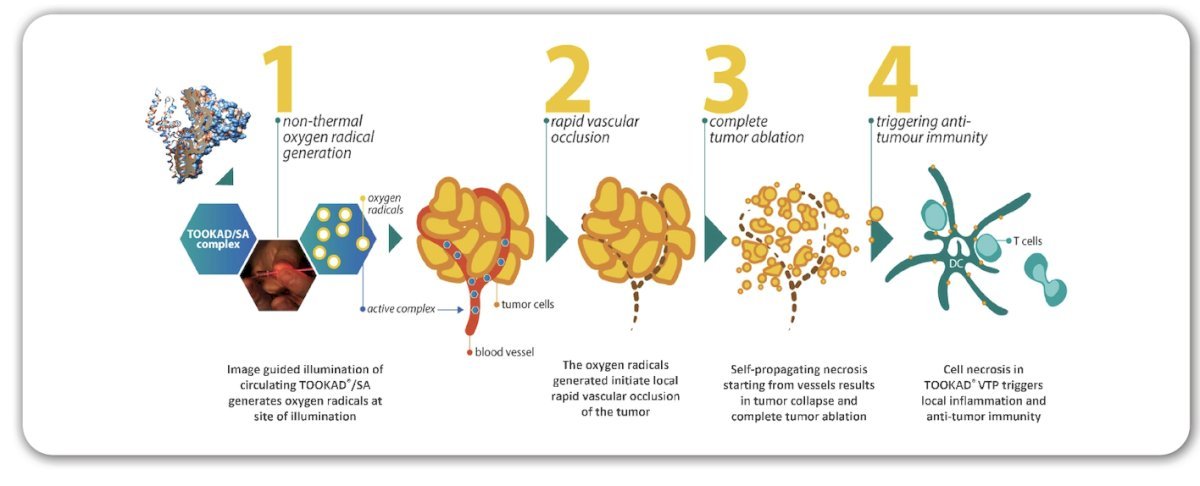
Upon laser light activation, padeliporfin triggers a cascade of events (non-thermal radical oxygen species generation, vascular occlusion, necrosis) that strongly impact tumor vasculature and induce an anti-tumor immune response:
Padeliporfin VTP has demonstrated safety and efficacy for upper tract urothelial carcinoma treatment in a phase 1 study (NCT03617003). At the SUO 2024 annual meeting, Dr. Margulis and colleagues reported the preliminary efficacy and safety outcomes of padeliporfin VTP for treatment of low grade upper tract urothelial carcinoma in ENLIGHTED, a phase 3 trial (NCT04620239).
The ENdoluminal LIGHT activated treatment of upper tract urothelial carcinoma (ENLIGHTED) trial is a single-arm, open-label, global pivotal phase 3 trial being conducted across 29 sites in the United States, Europe, and Israel. The target sample size is 100 patient enrollees, with 75 potentially evaluable. The ENLIGHTED study hypothesizes that padeliporfin VTP treatment effectively ablates low-grade upper tract urothelial carcinoma lesions, supporting the clinical goal of kidney preservation. This trial is divided into two phases: the induction and maintenance treatment phases. The induction treatment phase consists of 1-3 VTPs every 28 days. If complete response is not achieved after the first VTP, up to two additional VTPs are allowed. If complete response is achieved in the induction treatment phase, patients are allowed to enter the maintenance treatment phase (12 months). In the maintenance treatment phase, VTPs can be provided every 3 months for patients with recurrent tumor that is deemed treatable. A long-term follow-up phase is planned for patients completing the maintenance treatment phase. These patients will be followed for safety for 48 months with no VTPs.
This study included patients with new or recurrent low-grade, non-invasive upper tract urothelial carcinoma, meeting the following criteria:
- Male and female patients 18 years or older
- Up to two biopsy-proven low-grade UTUC tumors with the largest index tumor 5-15 mm in diameter (as measured by endoscopy), located in the calyces, renal pelvis, or in the ureter of the ipsilateral kidney
- Absence of high-grade cancer cells on cytology.
- Ureter involvement can be in one ureteral location (≤ 20 mm of contiguous ureteral length)
Key exclusion criteria included:
- Current high-grade or muscle-invasive (>pT1) urothelial carcinoma of the bladder
- Carcinoma in situ (CIS) – current or previous in the upper urinary tract
- History of invasive T2 or higher urothelial cancer in the preceding two years
- Prohibited medication that could not be adjusted or discontinued prior to study treatment
- Patients with photosensitive skin diseases or porphyria
The primary objective of this trial is to demonstrate the efficacy and durability of padeliporfin VTP and the secondary objectives included evaluating padeliporfin VTP-related safety and tolerability in treating low-grade upper tract urothelial carcinoma tumors in the kidney and ureter. Patients undergo initial screening to confirm eligibility, followed by induction treatment at 1–3 months thereafter. The study schema of ENLIGHTED is as follows:

As of July 22, 2024, out of the 32 patients treated, 20 patients completed induction treatment phase, and the complete response rate is 85%. The most frequent treatment related adverse events were (all grade 1-2, resolved within few days):
- Flank pain: 13%
- Hematuria: 7%
- Urinary tract infection: 7%
- Fatigue: 6%
- Procedural pain: 6%
- Nausea: 5%
- Vomiting: 5%
- Dysuria: 4%
- Pollakiuria: 4%
One patient experienced grade 3 serious adverse events solely related to the VTP, a renal colic resolved within 2 days. Padeliporfin VTP has shown evidence of safety and efficacy with preliminary data that is consistent with prior experience. Recruitment for the ENLIGHTED trial is ongoing with results expected to provide basis for approval of a new therapy that clinically benefits patients.
Presented by: Vitaly Margulis, MD, UT Southwestern University, Dallas, TX
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Society of Urologic Oncology (SUO) annual meeting held in Dallas, between the 3rd and 6th of December, 2024.


