(UroToday.com) At the Society of Urologic Oncology (SUO) Summer Virtual Webcast, Bishoy Faltas, MD, from Weill Cornell compared the genetic components of the bladder and upper tract urothelial carcinoma. Upper tract urothelial carcinoma comprises 5-10% of all urothelial carcinomas with a unique epidemiologic association with Arsenic exposure and aristolochic acid. Upper tract urothelial carcinoma presents with muscle invasion (pT2+) in 60% of cases, as compared to 20-30% of cases for bladder urothelial cancer. Upper tract urothelial carcinoma has more advanced disease at presentation but has similar outcomes to patients with bladder cancer after controlling for disease stage. It has been postulated that patients with upper tract urothelial carcinoma have more rapid invasion of the muscularis propria secondary to a thinner wall.
But is upper tract urothelial carcinoma and bladder urothelial carcinoma one or two diseases? Indeed, there are genomic differences between these two entities as highlighted in two recent European Urology articles.1-2 The Memorial Sloan Kettering cohort1 assessed tumor and germline DNA from patients with upper tract urothelial carcinoma (n=83) and bladder urothelial carcinoma (n=102), using a custom next-generation sequencing assay to identify somatic mutations and copy number alterations in 300 cancer-associated genes. Genes altered more commonly in high-grade upper tract urothelial carcinoma included FGFR3 (35.6% vs 21.6%; p=0.065), HRAS (13.6% vs 1.0%; p=0.001), and CDKN2B (15.3% vs 3.9%; p=0.016). Genes that were less frequently mutated in high-grade upper tract urothelial carcinoma included TP53 (25.4% vs 57.8%; p<0.001), RB1 (0.0% vs 18.6%; p<0.001), and ARID1A (13.6% vs 27.5%; p=0.050). The MD Anderson Cancer Center study collected 31 upper tract urothelial carcinoma samples and carried out whole-exome sequencing of DNA, RNA sequencing, and protein analysis. 2 Whole-exome sequencing identified mutations in FGFR3 (74.1%; 92% low-grade, 60% high-grade), KMT2D (44.4%), PIK3CA (25.9%), and TP53 (22.2%). Additionally, APOBEC and CpG were the most common mutational signatures.
Work from Dr. Faltas’ lab assessing genetics of upper tract urothelial carcinoma culminated in a Nature Communication publication last year,3 with several highlights. First, they noted that upper tract urothelial carcinoma is predominantly a luminal-papillary subtype, which was also confirmed in the Memorial Sloan Kettering Cancer Center (MSKCC) cohort. Second, upper tract urothelial carcinoma has a T-cell depleted immune contexture: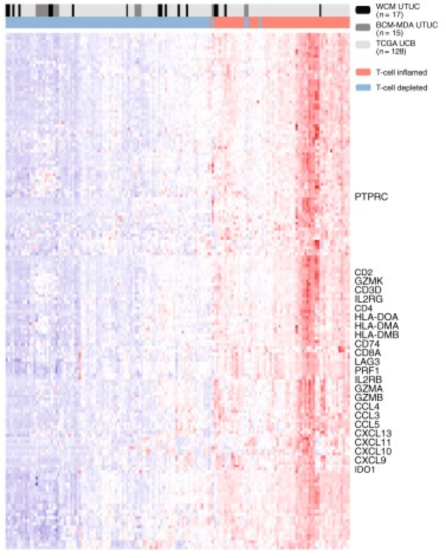
Third, high FGFR3 expression is enriched in upper tract urothelial carcinoma and correlates with its T-cell depleted immune microenvironment. Additionally, cells with FGFR3 knock out show upregulation of IFN-related genes, and erdafitinib upregulates IFN-related genes in urothelial cell lines with FGFR3 activating fusions. Finally, sporadic upper tract urothelial carcinoma is characterized by a lower total mutational burden than urothelial carcinoma of the bladder.
Upper tract urothelial carcinoma is one of the Lynch Syndrome defining cancers, and is the third most frequent malignancy, occurring in approximately 5% of patients. Patients with Lynch Syndrome have up to a 22-fold greater risk of developing upper tract urothelial carcinoma over the general population and a median age of onset of 10-15 years earlier than patients with sporadic upper tract urothelial carcinoma. As such, the majority of upper tract urothelial carcinomas are sporadic and not associated with Lynch Syndrome. Based on work from Dr. Faltas’ lab,3 MSI scores are not different between sporadic bladder urothelial carcinoma and sporadic upper tract urothelial carcinoma.
Recent work has also focused on defining the clonal relatedness of temporally distinct tumors with regards to bladder urothelial carcinoma and upper tract urothelial carcinoma.4 Compared with bladder urothelial carcinoma, TP53, RB1, and ERBB2 were less frequently altered in upper tract urothelial carcinoma (26% vs. 46%, 3% vs. 20%, 8% vs. 19%, respectively), whereas FGFR3 and HRAS were more frequently altered (40% vs. 26%, 12% vs. 4%, respectively). The risk of bladder recurrence after UTUC was significantly associated with mutations in FGFR3, KDM6A, CCND1, and TP53.
Dr. Faltas concluded his presentation with the following concluding remarks:
- Our understanding of upper tract urothelial carcinoma biology is rapidly advancing
- Most upper tract urothelial carcinomas are luminal-papillary and have a T-cell-depleted immune contexture
- Sporadic upper tract urothelial carcinomas do not have a high tumor mutational burden
- Bladder urothelial carcinoma recurrences after upper tract urothelial carcinoma are clonal ‘drop metastases’
- High FGFR3 expression is enriched in upper tract urothelial carcinoma and T-cell depleted immune microenvironment
- Future directions: upper tract urothelial carcinoma-targeted treatment strategies based on a deeper understanding of the biology
Presented by: Bishoy M. Faltas, MD, Director of Bladder Cancer Research, Englander Institute for Precision Medicine, Weill Cornell Medicine, New York, NY, USA
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Augusta, GA, USA, Twitter: @zklaassen_md at the Society of Urologic Oncology (SUO) - American Urologic Association (AUA) 2020 Summer Webcast Program, July 18, 2020.
References:
- Sfakianos JP, Cha EK, Iyer G, et al. Genomic Characterization of Upper Tract Urothelial Carcinoma. Eur Urol 2015 Dec;68(6):970-977.
- Moss TJ, Qi Y, Xi L, et al. Comprehensive Genomic Characterization of Upper Tract Urothelial Carcinoma. Eur Urol 2017 Oct;72(4):641-649.
- Robinson BD, Vlachostergios PJ, Bhinder B, et al. Upper tract urothelial carcinoma has a luminal-papillary T-cell depleted contexture and activated FGFR3 signaling. Nat Commun 2019 Jul 5;10(1):2977.
- Audenet F, Isharwal S, Cha EK, et al. Clonal relatedness and mutational differences between upper tract and bladder urothelial carcinoma. Clin Cancer Res 2019 Feb 1;25(3):967-976.


