Published Date: September 2019
Patients whose metastatic castration-resistant prostate cancer (mCRPC) has progressed on taxane chemotherapy and second-generation anti-androgen agents have few alternatives to palliative care. However, radiolabeled prostate-specific membrane antigen (PSMA) conjugates are now in latephase studies. In this article, I discuss theranostics, the phase 3 VISION trial, and the questions we will need to consider when PSMA-targeted radioligand therapies become available for use in our advanced prostate cancer clinics.
Theranostics in Prostate Cancer
In simple terms, theranostics combines a targeted therapeutic agent with a companion diagnostic that is used both to identify patients who are candidates for therapy and to assess treatment response. This union of drug therapy with diagnostics reflects recent progress in characterizing the various antigens expressed by prostate cancer cells, which shift in response to treatment and tumor-specific factors, as well as developing agents to specifically target these antigens. This is a highly personalized approach to medicine that supports more precise management of prostate cancer.
Gallium PSMA-based imaging identifies most prostate tumors sooner and with higher specificity compared with previous detection methods, which facilitate the earlier diagnosis and treatment of locally advanced and metastatic prostate cancer.1 However, imaging with a PSMA tracer also enables us to distinguish between patients with advanced prostate cancer who are, or are not, potential candidates for PSMA-directed radioligand therapies.2,3 Consider two patients whose scans both show metastatic prostate adenocarcinoma and whose disease is refractory to standard treatments. Patient A has a positive initial 68Ga-PSMA-11 PET/CT scan, indicating high expression of tumor cell membranous PSMA (mPSMA). Patient B has a negative 68Ga-PSMA-11 PET/ CT scan, indicating low or absent mPSMA expression. Clearly, PSMA-targeted therapy might make sense for patient A, but will not help patient B. If patient A decides to proceed with a PSMA targeted therapy, we can then perform follow-up 68Ga-PSMA-11 PET/CT scans to localize the location and determine the extent of high versus low PSMA-expressing disease, which will can help guide subsequent treatment decisions.4
177Lutetium is a beta-emitting radioisotope whose 6.7-day half-life is of sufficient duration for therapeutic use.5,6 Because the beta particles released by 177Lu travel less than 2 mm, they can irradiate small tumors and metastases—including micrometastases—at cytotoxic levels while minimizing damage to adjacent healthy tissues.6 177Lu also emits low-energy gamma particles, which are useful for performing imaging-based tumor localization and dosimetry before and during treatment. The 177Lu-labeled PSMA ligand that is furthest along in development is PSMA-617, a small molecule with high PSMA binding affinity. Since its initial characterization in 2015, 177Lu-PSMA-617 has produced objective responses with minimal toxicity in several (primarily small and retrospective) studies in Europe and Australia.4,7-10
In May 2018, investigators opened the prospective multicenter, randomized, phase 3 VISION trial (NCT03511664), which aims to enroll 750 patients with progressive mCRPC who have a positive 68Ga-PSMA-11 PET/CT scan and who have received at least one novel anti-androgen therapy (such as abiraterone or enzalutamide) and one to two prior taxane-based regimens.11 On a 2:1 basis, VISION patients are being randomly assigned to receive investigator-determined best supportive care, with or without or up to six cycles of 7.4 GBq (±10%) 177Lu-PSMA-617 (administered intravenously every 6 weeks, with response assessment at week 4). Primary endpoints are overall survival (OS) and radiographic progression-free survival (rPFS). Secondary endpoints include safety and tolerability, health-related quality of life, progression-free survival, prostate specific antigen (PSA) response, and health economics.
The VISION trial is being conducted at multiple centers in the United States as well as Puerto Rico, Canada, the United Kingdom, and Western Europe. VISION investigators aim to complete data collection for the primary outcome measures in May 2020.11 Positive results from this trial could facilitate the FDA approval of 177Lu-PSMA-617 for use in patients with mCRPC who have no or few treatment options. Participation in VISION also potentially enables U.S. patients with progressive, previously treated mCRPC to receive a treatment that has shown good tolerability and efficacy in Europe and elsewhere.
Alpha particle therapies that target PSMA are earlier in development but are poised to become important options for theranostics in the future. Alpha particles emit more energy than beta particles, and they do so over a significantly shorter distance. In preclinical and small clinical studies, targeted alpha therapy (TAT) agents such as 225Ac-PSMA-617, 225Ac-J591, and 227Th produced marked antitumor activity and strong PSA responses in patients with prostate cancer.12-16 Currently, a phase 1 multicenter study of the alpha particle emitter thorium-227 conjugated to a PSMAtargeted monoclonal antibody is enrolling in Louisiana, New York, Finland, and the United Kingdom (NCT03724747), while a phase 1 trial of 225Ac conjugated to the PSMA ligand J591 is recruiting at Weill Cornell Medical College in New York City (NCT03276572). Although xerostomia is a frequent side effect of PSMA-targeted alpha emitters, they nonetheless may someday supplant 177Lu-PSMA-617 in carefully selected patients, or it may be used to treat the subset of patients who do not respond to 177Lu-labeled PSMA ligand treatments.17
Next, I will discuss the lingering questions that need to be addressed after theranostics joins our prostate cancer armamentarium.
Patient Selection
While membranous prostate-specific membrane antigen (mPSMA) expression tends to be upregulated in mCRPC, its expression varies considerably both between and within patients. Clearly, there is no indication for PSMA-targeted therapy if a patient’s prostatic adenocarcinoma is found to be low mPSMA-expressing (that is, the patient has a negative 68Ga-PSMA PET/ CT scan). However, the cellular and molecular heterogeneity of prostate cancer means that not all cases are clear-cut.
In one recent study, for example, researchers used immunohistochemistry to score mPSMA expression in 38 patients with advanced prostate cancer and then correlated these scores with clinical outcomes and the results of next-generation sequencing (NGS).18 Higher mPSMA expression at diagnosis correlated with higher Gleason grade (P=.04) and worse overall survival (OS) (P=.006). Expression of mPSMA was undetectable in 42% of hormone-sensitive tumor specimens and 27% of castration-resistant tumor specimens. However, mPSMA expression varied markedly both within and among tumor specimens. In patients with mCRPC, immunohistochemistry identified PSMA-positive and PSMA-negative cells interspersed within single areas of prostate tumor, clusters of closely entwined high-PSMA and nonPSMA expressing tumor cells, and areas of PSMA-negative tumor cells located more than 2 mm away from regions of high-PSMA expressing cells. Since the maximum 177Lu tissue penetrance is 2 mm, 177Lu-PSMA therapy could not be expected to destroy all PSMA-negative cells in these latter patients.
Such extensive heterogeneity of intra-patient mPSMA expression complicates patient selection for PSMA-targeted therapy. Because our candidates for theranostics will have treatment-refractory mCRPC, it is vital to identify accurate biomarkers of clinically relevant responses to theranostics. One possibility is DNA damage repair (DDR) mutations. In the immunohistochemistry study, patients with high-mPSMA expressing mCRPC were significantly more likely to have alterations in BRCA2 and ATM.18 A recent case report of a patient with a BRCA2 mutation documented a remarkable response to 177Lu PSMA-targeted therapy,19 while a retrospective whole-exome sequencing study of mCRPC patients receiving PSMA-targeted radionuclide therapy reported a median OS of 49 months in the subgroup with BRCA2 aberrations versus 17 months in BRCA2 wild-type patients (P=.09).20 Among the 57% of patients who received 177Lu-PSMA-617 in this study, BRCA2 aberrations were associated with a significantly longer OS even after accounting for possible confounders, such as low-risk versus high-risk disease status (hazard ratio, 0.1; 95% CI, 0.02 to 0.42; P=.002).
Sequencing and Layering
The plethora of new approvals in advanced prostate cancer inevitably raises questions about how to sequence and layer therapies. We lack informative data from randomized clinical trials, because most studies of PSMA-targeted theranostics have enrolled patients with few or no other treatment options. Current guidelines from the American Urological Association (AUA) and the National Comprehensive Cancer Network (NCCN) also do not discuss theranostics or PSMA-targeted radionuclide therapy as they are not currently FDA-approved.21,22 We can expect these and other guidelines to be revised to reflect the results of ongoing and future trials. For the time being, I support the general recommendation that clinicians should consider performance status, symptoms, comorbidities, disease volume, and prior treatment response(s) when sequencing treatments for advanced prostate cancer.23 Cost and patient preference will also play an important role in clinical decision-making.
What about combination therapies with PSMA-targeted radionuclides? The researchers who led the small immunohistochemistry study hypothesized that mPSMA expression might be dynamically regulated, and that prostate tumor cells with DNA damage repair mutations might exhibit high mPSMA expression in response to replication stress in.18 If future studies support this premise, then it might make sense to look at combining PSMA-targeted therapies with treatments that promote tumor cell replication stress or genomic instability.
Several ongoing studies are evaluating theranostics head-to-head and in combination with other treatments for mCRPC. In Australia, researchers are enrolling a phase 2 head-to-head trial of 177Lu-PSMA-617 versus cabazitaxel (NCT03392428), a phase 1 trial of 177Lu-PSMA-617 in combination with the poly ADP ribose polymerase (PARP) inhibitor olaparib (NCT03874884), and a phase 1/2 trial of 177Lu-PSMA-617 in combination with the anti-programmed cell death (PD-1) antibody pembrolizumab (NCT03658447). In the United States, phase 1 and phase 2 trials are evaluating docetaxel-prednisone plus fractionated 177Lu conjugated to the PSMA-targeting antibody J591 (NCT00916123), 177Lu-PSMA-617 plus the PD-1 inhibitor pembrolizumab (NCT03805594), and fractionated-dose 177Lu-J591 plus 177Lu-PSMA-617 (NCT03545165). Hopefully, the results of these studies will help guide future decisions regarding theranostic sequencing and layering.
Treatment Response
Molecular characterization of advanced prostate cancer not only helps identify candidates for targeted therapy but also enables us to monitor treatment response and make subsequent treatment decisions. Recently, researchers examined 177Lu-PSMA-617 therapy in 14 men with progressive, symptomatic, PSMA-positive mCRPC who had progressed on anti-androgen therapy (and also, in some cases, on taxane-based chemotherapy).4,24 The results of this small study illustrate the anti-tumor activity of 177Lu-PSMA-617 in carefully selected patients with mCRPC. Among 10 (71%) patients with a PSA response (mean 59% reduction in PSA), seven patients also showed a soft tissue response, based on computed tomography (CT) RECIST assessment.4 Soft-tissue responders survived a median of 17 weeks longer than did soft-tissue nonresponders.4
In this study, the intensity of PSMA activity predicted treatment response even though all patients had baseline PSMA PET activity above or equal to liver and no regions of FDG-PSMA mismatch.4 Patients whose PSA levels declined by at least 30% after PSMA-Lu therapy had significantly higher maximum and mean PSMA uptake compared with PSA nonresponders (P<.007 and P<.04, respectively). No patient with a PSA response had a PSMA SUVmax of less than 15, and the higher the ratio of PSMA SUVmax to FDG SUVmax, the greater the likelihood of biochemical response.
Furthermore, PET imaging by PSMA and FDG identified distinct patterns of treatment failure among men who experienced PSA progression during or after Lu-PSMA therapy. Table 1 shows how these findings were used to guide subsequent treatment.24
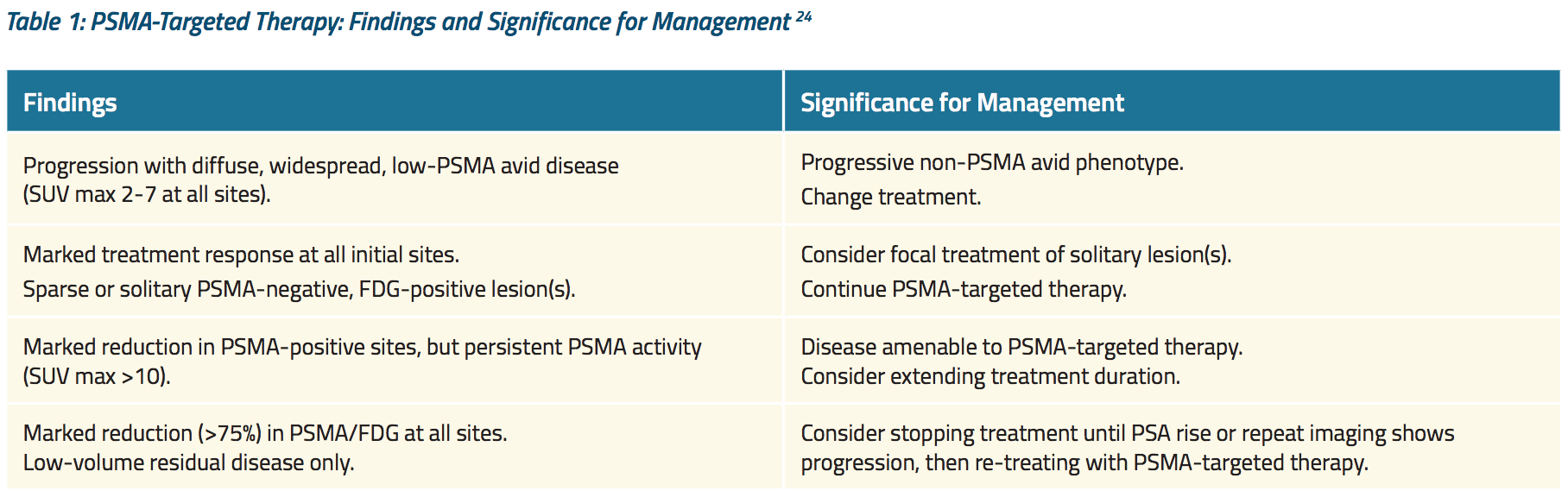
Until now, studies like this one have used PSMA-based imaging to assess treatment response. Some experts have criticized this practice: If a patient is receiving a PSMA-targeted therapy, is a negative PSMA scan really a good indicator of disease control? As we have seen, prostate cancer is heterogeneous — what if the patient is experiencing progression in low-PSMA expressing cell lines? Some experts argue for using a metabolic tracer such as fluciclovine. Others contend that imaging does not add clinically meaningful insights beyond what we obtain from monitoring PSA and symptoms.
In my view, the heterogeneity of mPSMA expression in advanced prostate cancer will necessitate a thoughtful approach to monitoring response to PSMA-targeted radionuclide therapy. Monitoring of PSA and clinical assessment will be the foundation, but imaging will play a role, since we know that a significant portion of patients will progress without an accompanying rise in PSA.25 To better assess tumor heterogeneity, we may need to routinely incorporate more than one type of imaging, such as CT, FDG PET/CT, and PSMA PET/CT.
In the future, we can expect to see more studies of the best ways to use molecular imaging to assess response and guide treatment. Treatments may also include targeted external beam radiotherapy of low PSMA-expressing lesions in patients who are otherwise responding. As always, obtaining useful insights from clinical trials and observational studies will require multidisciplinary input into study designs and thoughtful communication between imagers and clinicians. This was the conclusion reached by urologists, oncologists, radiologists, and nuclear medicine specialists during a recent consensus meeting on the role of molecular imaging in prostate cancer theranostics.26
Radiation Safety
Theranostics based on radionuclide therapy will inevitably raise questions regarding radiation safety. The central principle when working with ionizing radiation is to keep exposure to staff, patients, and their close contacts As Low as Reasonably Achievable (ALARA). Most patients will receive 177Lu-PSMA in a nuclear medicine clinic that already follows standard ALARAbased protocols and practices. However, some prostate cancer clinics will incorporate nuclear medicine into their practices, and all clinicians should be ready to discuss radiation safety with staff, patients, and caregivers.
177Lu-PSMA is excreted renally, and the majority of excretion occurs during the first 48 hours after infusion.9 Patients, staff, and caregivers therefore need to know how to manage radioactive spills.27 Standard bathroom precautions include flushing multiple times after voiding, wearing gloves to clean up any spills, and practicing hand washing and other good hygiene measures.28 Clinical experience in Australia suggests that patients emit less than 25 μSv ionizing radiation per hour beginning one hour after 177Lu-PSMA treatment.27 As a precaution, however, it is reasonable to require patients to remain at the nuclear medicine clinic for 2-4 hours post-treatment.27
In a recent study, researchers measured radiation exposure to staff and the close family members of 23 patients who received 177Lu-PSMA at a mean dose of 7500 Mbq per intravenous infusion, which is 100 Mbq higher than the dose used in the ongoing VISION trial.29 In the study, all members of the medical team— physicians, nurses, and nuclear medicine technologists—received mean dose rates under 25 μSv/h (between 2 and 6 μSv per patient). The total mean dose for caregivers was 202.3 μSv over five days (range, 120 to 265 μSv). These findings suggest that 177Lu-PSMA can be safety administered in outpatient settings with standard radiation precautions.
In another recent study of 50 patients who received 177Lu-PSMA-617 (mean administered activity, 6.3±0.5 GBq), approximately 50% of this agent was excreted after 4 hours and approximately 70% was excreted after 12 hours.30 The maximum dose per treatment cycle to caregivers and close relatives was approximately 250±55 μSv when the patient was discharged after 48 hours and approximately190±36 μSv when the patient was discharged after 72 hours. The researchers concluded that 177Lu-PSMA-617 is a safe option for therapy, but that it should be administered in a way that avoids exceeding legal limits for radiation exposure to individuals, as well as excretion into wastewater. Such findings confirm the importance of strong radiation safety programs when using this radionuclide therapies.
Notwithstanding the good overall safety profiles of PSMA-targeted radiopharmaceuticals, physiologic uptake by normal tissue occurs and also must be kept in mind.9,27 With 177Lu-PSMA-617, the salivary glands receive the highest radiation dose, while renal uptake is minimal.31 The use of imaging to guide patient-specific dosimetry can help minimize unnecessary irradiation of normal tissue. Bone marrow toxicity is unusual with 177Lu-PSMA-617, but it can occur in the setting of extensive bone metastases and borderline bone marrow function.9,27 Again, upfront PSMA imaging can help identify patients at risk for higher-grade myelosuppression.
Alpha particle-emitting radiotherapies such as radium-223 travel shorter distances than their beta-particle counterparts. Hence, alpha particle therapies such as 225Ac-PSMA-217 may make sense for patients at increased risk of myelosuppression. Current federal regulations specify how alpha-emitting radionuclides are to be handled with regards to packaging, transport, and surface decontamination.32 However, federal regulations do not mandate contact precautions for clinical use and require only one week of bathroom precautions following treatment.29 This reflects the good safety profile of targeted alpha radionuclide therapies.
The bottom line when we consider radiation safety is multidisciplinary care. Both urologists and medical oncologists will benefit from identifying nuclear medicine and radiation oncology specialists who are comfortable performing these therapies for patients with advanced prostate cancer, committed to maintaining good working relationships with referring providers, and accustomed to educating patients and caregivers about radiation safety practices during and after treatment.
Summary
Theranostics based on PSMA are poised to transform the assessment and treatment of patients with advanced prostate cancer. The international, phase 3 VISION trial is evaluating radiographic progression-free survival and overall survival with 177Lu-PSMA-617 therapy in men whose PSMA-positive, progressive mCRPC is refractory to standard therapy. Positive safety and efficacy results from this trial could lead to a first-in-class approval for prostate cancer theranostics in the United States. While 177Lu is a beta particle emitter, PSMA-targeted alpha therapies have also shown promise and may someday join our armamentarium. Because prostate tumors show heterogeneous levels and patterns of mPSMA expression, PSMA-based imaging helps identify candidates for PSMA-targeted therapy and also can help monitor treatment response. Evidence suggests that prostate cancer patients with certain DNA damage repair mutations are more likely to respond to 177Lu-PSMA-617, but additional biomarkers are needed to guide patient selection. Ongoing studies that compare or combine theranostics with other treatments will help guide therapeutic sequencing and layering. As always, clinicians will need to carefully factor patient and disease-specific factors into these decisions. Staff should follow standard radiation safety protocols that keep ionizing radiation exposure as low as reasonably achievable (ALARA) before, during, and after using theranostics.
Written by: Phillip J. Koo, MD, is the Division Chief of Diagnostic Imaging at the Banner MD Anderson Cancer Center in Arizona. Prior to this, he was Chief of Nuclear Medicine and Associate Professor of Radiology at the University of Colorado School of Medicine. Dr. Koo completed his transitional internship at the University of Pennsylvania Medical Center-Presbyterian, radiology residency at Pennsylvania Hospital of the University of Pennsylvania Health System, and fellowship at the Harvard Medical School Joint Program in Nuclear Medicine. He is a diplomate of both the American Board of Radiology (ABR) and American Board of Nuclear Medicine. Dr. Koo is an active member of multiple societies and serves on the scientific program committee for the Radiological Society of North America, nuclear radiology certifying exam committee for the ABR, and is the Chair of the Quality and Evidence Committee for the Society of Nuclear Medicine and Molecular Imaging.
References:
1. Maurer T, Gschwend JE, Rauscher I, et al. Diagnostic efficacy of (68)gallium-psma positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol. 2016;195(5):1436-1443.
2. Yordanova A, Eppard E, Kürpig S, et al. Theranostics in nuclear medicine practice. Onco Targets Ther 2017 Oct;10:4821-4828.
4. Emmett L, Crumbaker M, Ho B, et al. Results of a prospective phase 2 pilot trial of Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer including imaging predictors of treatment response and patterns of progression. Clin Genitourin Cancer. 2019;17(1):15-22.
5. Pommé S, Paepen J, Altzitzoglou T, et al. Measurement of the 177Lu half-life. Appl Radiat Isot. 2011;69(9):1267-1273.
6. Dash A, Pillai MR, Knapp FF. Production of (177)Lu for targeted radionuclide therapy: available options. Nucl Med Mol Imaging. 2015;49(2):85-107.
8. McBean R, O’Kane B, Parsons R, et al. Lu177-PSMA therapy for men with advanced prostate cancer: Initial 18 months experience at a single Australian tertiary institution. J Med Imaging Radiat Oncol. 2019 Apr 25. doi: 10.1111/1754-9485.12891. [Epub ahead of print]
10. Ahmadzadehfar H, Wegen S, Yordanova A, et al. Overall survival and response pattern of castration-resistant metastatic prostate cancer to multiple cycles of radioligand therapy using [Lu] Lu-PSMA-617. Eur J Nucl Med Mol Imaging. 2017;44(9):1448-1454.
12. Kratochwil C, Bruchertseifer F, Giesel FL, et al. 225Ac-PSMA-617 for PSMA-Targeted a-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J Nucl Med 2016 Dec;57(12):1941- 1944. Epub 2016 Jul 7.
13. Kratochwil C, Bruchertseifer F, Rathke H, et al. Targeted α-therapy of metastatic castration-resistant prostate cancer with Ac-PSMA-617: swimmer-plot analysis suggests efficacy regarding duration of tumor control. J Nucl Med. 2018;59(5):795-802.
15. van der Doelen MJ, Niven Mehra, Minke Smits, et al. Clinical experience with PSMA-Actinium-225 (Ac-225) radioligand therapy (RLT) in end-stage metastatic castration-resistant prostate cancer (mCRPC) patients. J Clin Oncol 2018;36, no. 6_suppl:344-344.
16. Hammer SLA, Ellingsen C, Geraudie S, et al. Preclinical pharmacology of the PSMA-targeted thorium-227 conjugate PSMA-TTC: A novel targeted α therapeutic for the treatment of prostate cancer; Proceedings of the AACR Annual Meeting 2017; Washington, DC, USA. 1–5 April 2017.
19. Crumbaker M, Emmett L, Horvath LG, et al. Exceptional response to 177Lutetium prostate-specific membrane antigen in prostate cancer harboring DNA repair defects. JCO Precis Oncol 2019 Feb 6. DOI: 10.1200/PO.18.00237
20. Panagiotis JV, Conteduca V, Hackett AL, et al. Prognostic value of BRCA2 and AR gene alterations in advanced prostate cancer patients treated with PSMA-targeted radionuclide therapies [abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2019; 2019 Mar 29-Apr 3; Atlanta, GA. Philadelphia (PA): AACR; Cancer Res 2019;79(13 Suppl):Abstract nr 4865.
21. Ezez. 2019. . Nccn.org. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
22. American Urological Association. Castration-resistant prostate cancer (2018). https://www.auanet.org/guidelines/prostate-cancer-castration-resistant-guideline Accessed July 31, 2019.
24. Joshua AM, Stricker P, Epstein RJ, et al. Patterns of failure on Ga PSMA (GaPSMA) and F18 FDG (FDG) PET CT in a prospective phase 2 trial of 177Lu DKFZ PSMA 617 (LuPSMA) in men with castrate resistant metastatic prostate cancer (mCRPC). J Clin Oncol. 2017;35(15_suppl):2562-2562.
25. Bryce AH, 2017, et al. Radiographic progression with nonrising PSA in metastatic astration-resistant prostate cancer: post hoc analysis of PREVAIL. Prostate Cancer Prostatic Dis. 2017 Jun;20(2):221-227
29. Demir M, Abuqbeitah M, Uslu-beşli L, et al. Evaluation of radiation safety in (177)Lu-PSMA therapy and development of outpatient treatment protocol. J Radiol Prot. 2016;36(2):269-278.
30. Kurth J, Krause BJ, Schwarzenböck SM, et al K. External radiation exposure, excretion, and effective half-life in Lu-PSMA-targeted therapies. EJNMMI Res. 2018;8(1):32.
32. U.S. Nuclear Regulatory Commission. Program specific guidance about medical use licenses. Vol. 9. Washington, DC: Division of Industrial and Medical Nuclear Safety. Office of Nuclear Material Safety and Safeguards; United States Nuclear Regulatory Commission; 2008. Consolidated guidance about materials licenses. Final report; NUREG-1556. Rev. 2
Remaining sources:
Seidlin SM, Marinelli LD, Oshry E. Radioactive iodine therapy; effect on functioning metastases of adenocarcinoma of the thyroid. J Am Med Assoc. 1946;132:838-847.
Ezez. 2019. . Nccn.org. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.


