Introduction
Intravesical Bacillus Calmette-Guerin (BCG) currently remains the standard of care, guideline recommended treatment of choice in the adjuvant setting for intermediate- and high-risk non-muscle invasive bladder cancer (NMIBC) due to its ability to reduce the risk of disease recurrence and disease progression.1-3 However, despite adequate BCG, up to 50% of patients develop a BCG-refractory, relapsing, or failure disease state.4 Currently, radical cystectomy remains the gold standard approach in this setting.1 However, many patients are either unfit or refuse cystectomy.
Given the shortage of options in this disease space, along with the modest efficacy of Valstar (21% complete response with a median response duration of one year),5 in 2018 the FDA issued a guidance document to promote the development of drugs and biologics for BCG-unresponsive NMIBC:6
- Persistent or recurrent CIS within 12 months of adequate BCG therapy
- Recurrent high-grade Ta/T1 disease within six months of completion of adequate BCG therapy
- High-grade T1 disease detected on the first evaluation following an induction BCG course
The focus of this Center of Excellence article is to highlight the currently available evidence and ongoing studies for Nogapendekin alfa-inbakicept (NAI; Anktiva, ie. N-803) in NMIBC in the BCG-unresponsive disease space, as well as other ongoing trials in progress for other indications.
QUILT-3.032: Initial Results
Impaired T-cell and cytotoxic cellular response play a key role in the development of BCG failure for patients with NMIBC. N-803 is an interleukin-15 (IL-15) superagonist that acts as an activation and proliferation factor for natural killer and effector/memory T-cells:

Given that BCG has been shown to establish ‘trained immunity’ as the molecular basis for its immunotherapeutic effect in bladder cancer, it has been hypothesized that N-803 could further enhance the immune response by mediating a second, unrelated stimulus.
QUILT-3.032 is an ongoing, open label, multicenter, single arm trial that is evaluating intravesical N-803 + BCG or N-803 alone in patients with BCG-unresponsive, high-grade NMIBC7. This study includes three patient cohorts8:
- Cohort A (CIS +/- papillary disease): Intravesical N-803 (400 μg/instillation) + BCG (50 mg/installation) given once weekly for 6 consecutive weeks (induction)
- Cohort B (High grade Ta/T1 papillary disease): N-803 + BCG
- Cohort C (CIS +/- papillary): N-803 alone

In this trial, surveillance included a cystoscopy + urinary cytology every 3 months for the first 24 months, followed by every six months thereafter until 60 months. Importantly, biopsy was mandated at week 12 in all patients, irrespective of cystoscopy or cytology findings. Re-induction was offered to patients without a complete response, conditional on the absence of T1 or worse disease in the specimen. These patients would then undergo a mandatory 6-month biopsy. The primary endpoints in Cohorts A and C were 3- and 6-month complete response, defined by one of the following three scenarios:
- Negative cystoscopy and cytology (including atypical cytology)
- Biopsy-proven benign or low-grade Ta disease and negative cytology
- Negative cystoscopy with malignant urinary cytology, if cancer found in the upper tract or prostatic urethra and random bladder biopsies were negative
In Cohort A (n = 82), a complete response was observed in 71% of patients (13/58 responders required a re-induction). Response rates were as follows:
- 3 months: 55%
- 6 months: 56%
- 12 months: 45%
The median duration of response in complete responders was 26.6 months. The 24-month progression-free, overall, and disease-specific survival rates were 85%, 94%, and 100% respectively.

In 72 evaluable patients in Cohort B, the median disease-free survival was 19.3 months (95% CI 7.4 – NR), with disease-free survival rates as follows:
- 12 months: 55.4% (95% CI 42.0 – 66.8)
- 18 months: 51.1% (95% CI 37.6 – 63.1)
- 24 months: 48.3% (95% CI 34.5 – 60.7)

The 24-months progression-free, overall, and disease-specific survival rates were 88.8%, 91.7%, and 97.7%, respectively.
In the 10 evaluable patients in Cohort C, a complete response at 3 months was observed in only 2 (20%) patients with N-803 alone. Of the 8 initial non-responders, 6 underwent re-induction with only 1 of these 6 patients demonstrating evidence of a complete response at 6 months. On the basis of protocol-defined stopping rules, the independent data monitoring committee recommended that cohort C be discontinued for futility.
The most common treatment-related adverse events with the combination of N-803 + BCG were lower genitourinary in nature (dysuria, pollakiuria, hematuria). The incidence of grade 3 or worse adverse events was 23%, most frequently hematuria and urinary tract infections (2% each). One patient experienced a grade 5 treatment-related adverse event (cardiac arrest with subsequent death) and 3 had evidence of immune-related adverse events:7
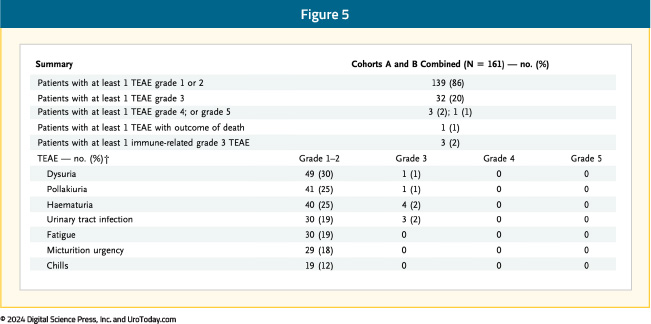
QUILT-3.032: After the Initial Results
FDA Approval
On April 22, 2024, the Food and Drug Administration (FDA) approved N-803 + BCG for patients with BCG-unresponsive NMIBC with CIS +/- papillary tumors based on results from the QUILT-3.032 trial.
Quality of Life Assessment
In Cohort A, the mean baseline ECOG score was 0.183, with 82% of patients having a score of 0. Quality of life was measured by the EORTC QOL Questionnaire Core 30 (QLQ-C30) and QOL NIMBC-Specific 24 Questionnaire (QLQ-NMIBC24). During the study, hospitalizations for any reason remained low (0% - 6% per assessment). Moreover, participant quality of life from completion rate was high, with 90% or greater at most time points. There was a modest decrease in mean physical function and global health from baseline at all assessed on-study time points that became less by week 104: 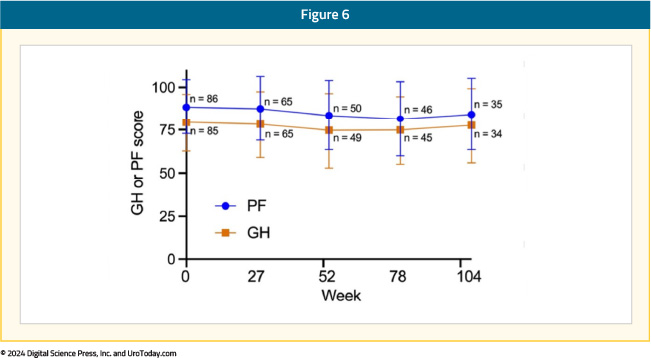
When responders (those with a complete response) were compared with non-responders, they showed less of a decrease in physical function and global health scores with time, although both parameters were higher at baseline for responders:

At month 12, >3 prior TURBTs were significantly associated (p = 0.0729) with lower global health scores as compared to <= prior TURBTs. At month 6, achievement of complete response was associated (p = 0.0659) with higher physical function scores as compared to no complete response. No other baseline variable had a significant level of association:

QUILT-3.032 Updated Results
At AUA 2024, Dr. Patrick Soon-Shiong presented updated results of the QUILT-3.032 trial. At the time of the data cutoff of November 30, 2023, patients with an ongoing complete response at month 25 and later, maintenance instillations were administered once a week for 3 weeks at months 25, 31, and 37 for a maximum of 9 additional instillations. In this highly pre-treated group, the complete response rate was 65%. Among patients who had received ≥12 prior BCG doses (n = 74), the complete response rate was 69%. Conversely, among those who received <12 doses of prior BCG, the complete response rate was 70%. A total of 53 patients were BCG-unresponsive/BCG-relapsed, and the complete response rate in this group was 72%. Thus, both BCG-unresponsive/BCG-refractory and BCG-unresponsive/BCG-relapsed had complete response rates comparable to the efficacy population. The median duration of response was 26.6 months (95% CI: 0.9 – not reached). By the Kaplan-Meier method, 61.3% of subjects had a duration of complete response lasting >12 months and 53.2% had a duration of >24 months.
Looking Ahead: QUILT-2.005 in BCG-Naïve Patents
Also at AUA 2024, Dr. Sandeep Reddy discussed the QUILT-2.005 trial comparing intravesical BCG in combination with N-803 to BCG alone in patients with BCG-naïve NMIBC. In phase 1 of this trial, there was durable complete remission among 9 patients with CIS and papillary NMIBC treated with BCG + N-803. As requested by the Agency, there was an unplanned interim analysis of the efficacy results in CIS patients for the phase 2 QUILT-2.005 trial, which showed an improvement in complete response rate over time and contribution of effect of N-803 inducing memory T-cells
The trial design for the phase IIb QUILT-2.005 is for participants to be randomized 1:1 to one of two independent treatment arms based on disease and ECOG status. Participants receive either intravesical 400 ug N-803/instillation + 50 mg BCG or BCG alone weekly for six consecutive weeks, followed by maintenance treatment for 3 consecutive weeks at 3, 6, 12, and 18 months. At 3 months, re-induction with another 6 weeks of therapy for eligible patients is an option. Cohort A of QUILT-2.005 will have CIS +/- Ta or T1 disease patients, and Cohort B will have high grade papillary Ta or 1 disease patients. Participants must have a diagnostic biopsy within 3 months of treatment start and a cystoscopy demonstrating no resectable disease within 6 weeks. Participants must be BCG-naïve (or last BCG > 3 years prior) and have not received more than a single post-operative dose of mitomycin C or gemcitabine following the most recent screening TURBT/biopsy.
The primary outcomes of QUILT-2.005 are complete response rate and disease free survival with the time frames of 12 and 24 months, respectively. The complete response rate for Cohort A and disease free survival for Cohort B will be compared between treatment arms using cystoscopy, confirmatory biopsy and urine cytology. Safety will be assessed by number/severity of treatment related adverse events over 48 months. As of April 2024, cohort A had enrolled 121 of 366 planned participants, and cohort B had enrolled 74 of 230 planned participants.
N-803 + BCG Logistics and Insurance Coverage
In May 2024, it was announced that ImmunityBio had signed a global agreement with Serum Institute of India (the world’s largest manufacturer of vaccines) to supply ImmunityBio with BCG. The agreement covers manufacturing of standard BCG (approved outside of the US), as well as next generation recombinant BCG (undergoing testing), for the intended use in combination with N-803. Importantly, the aim of this deal is to increase the available supply of BCG and address shortages for the combination with N-803. Furthermore, there are plans to conduct clinical trials to study recombination BCG and standard BCG manufactured by Serum Institute in combination with N-803 for NMIBC.
As of August 2024, N-803 is approved for coverage by more than a dozen insurance plans (corresponding to ~100 million individuals), only months after the FDA approval in the US. Additionally, ImmunityBio has filed for EMA regulatory approval in the European Union, as well as approval to enroll patients in India for the QUILT-2.005 trial. Plans are also underway to apply for approval in South Africa to enroll patients in QUILT-2.005.
Conclusions
Results from the QUILT-3.032 trial in BCG-unresponsive NIMBC have led to the FDA approval of N-803 + BCG in 2024. Importantly, this combination therapy is now approved by several insurance plans and has been recognized as a therapeutic option in the NCCN guidelines. In light of the global BCG shortages, ImmunityBio has successfully partnered with Serum Institute of India to increase BCG availability. Results of the ongoing QUILT-2.005 trial in BCG naïve NMIBC are eagerly anticipated, with continued site expansion in the US, as well as India and potentially South Africa.
Published November 2024


