From May 2020 to June 2022, monthly surveys were distributed to lead investigators of the CISTO network, which is involved in a pragmatic observational trial comparing radical cystectomy and bladder-sparing therapy for recurrent high-risk non-muscle-invasive bladder cancer (NMIBC). The survey aimed to assess the continuation of various clinical activities, including elective surgeries, transurethral resection of bladder tumors, adjuvant intravesical therapies, surveillance cystoscopy, and radical cystectomy, as well as the availability of intravesical bacillus Calmette-Guerin (BCG), during the COVID-19 PHE.
In all, 32 CISTO study sites were included, spanning academic, community, and international practices during the study period. The geography of these sites were as follows: 5 in the West, 5 in the Midwest, 8 in the South, 2 in the Northeast, and 1 international site. The surveys revealed that 76% of the responding sites experienced pauses or decreases in general elective surgery starting in May 2020, with a reduction in restrictions by July/August 2020. However, elective surgery limitations increased again during the winter of 2020-2021, peaking in January 2021 with 48% of sites affected, likely due to the COVID-19 delta variant, and saw another rise in restrictions during the fall/winter of 2021 corresponding with the emergence of the omicron variant.
During the pandemic, interruptions in bladder cancer surgeries, such as TURBT and radical cystectomy, were initially observed and subsequently aligned with the emergence of COVID-19 variants. Specifically, TURBT was limited at 14% of CISTO sites in early 2021, while restrictions on radical cystectomy were noted at 10-14% of sites at the end of 2020 and beginning of 2021, with further limitations in the fall of 2021. Office-based cystoscopies faced a significant reduction in May 2020, with 33% of sites experiencing limitations, but saw fewer disruptions afterwards. The extent of these restrictions varied across US regions, with the Northeast experiencing more significant interruptions in bladder cancer care. Additionally, the availability of intravesical BCG was inconsistent, with over a third of sites facing shortages in mid-2021 and ongoing limitations for about 20% of sites throughout the two-year period.
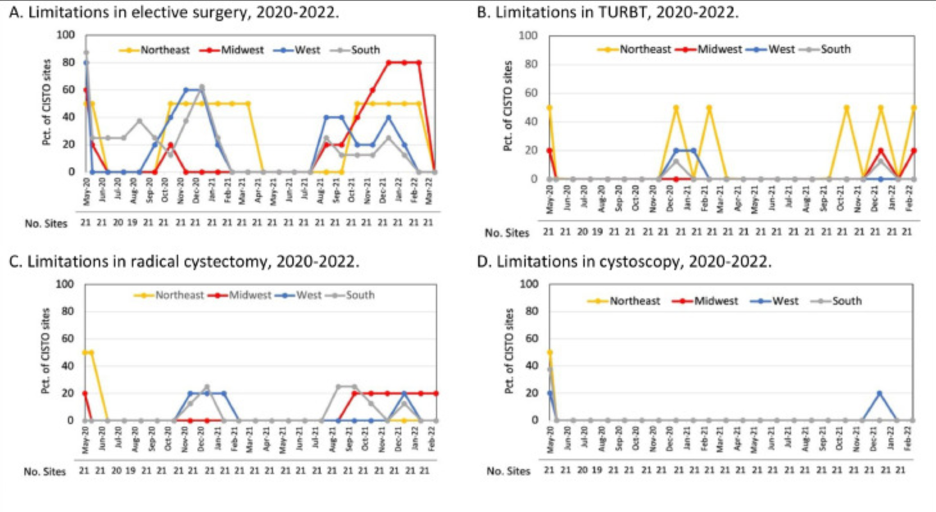
The authors conclude that, within a wide-ranging network of sites, disruptions to bladder cancer care were relatively minimal in comparison to the more extensive restrictions applied to elective surgeries during the COVID-19 pandemic. Additionally, the high risk NMIBC population was additionally facing a BCG-shortage, which compounded barriers to care. The continuation of necessary surgical treatments for bladder cancer patients was highlighted as a positive finding; however, as Gore et al. conclude, subsequent analyses will be important to determine if this consistent care results in maintained health outcomes for patients treated for bladder cancer during the studied period.
Written by: Ruchika Talwar, MD, Urologic Oncology Fellow, Department of Urology, Vanderbilt University Medical Center, Nashville, TN
References:
Published February 2024


